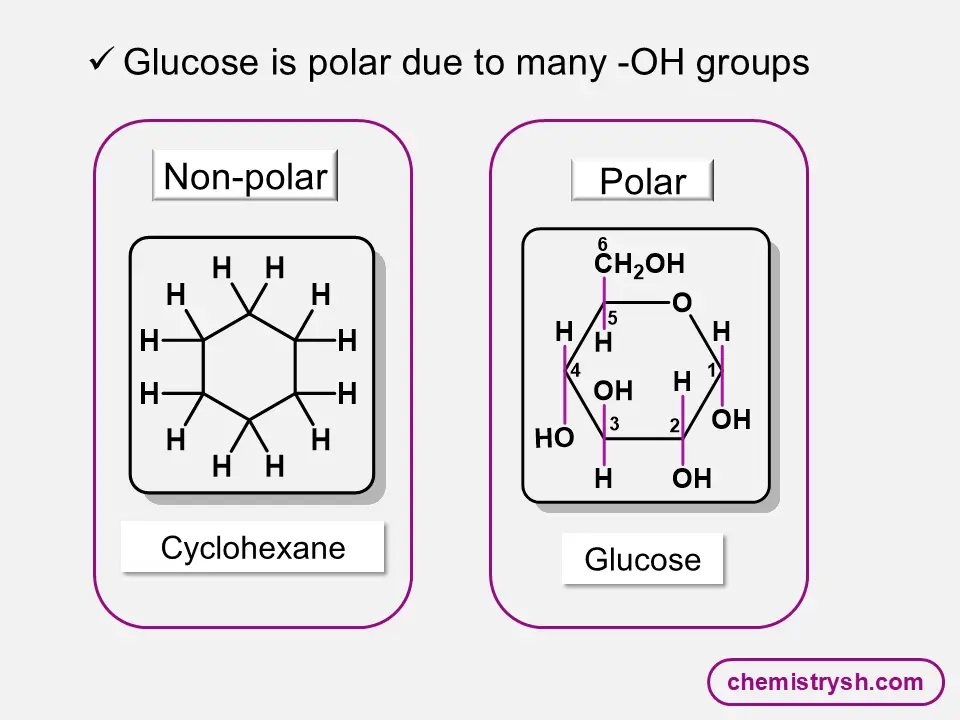
What Is Solubility?
Solubility is the maximum amount of a substance (solute) that can dissolve in a given amount of a solvent at a specific temperature (and pressure) to form a homogeneous solution.
In simple words, solubility tells us how much of a substance can dissolve in a solvent like water under fixed conditions.
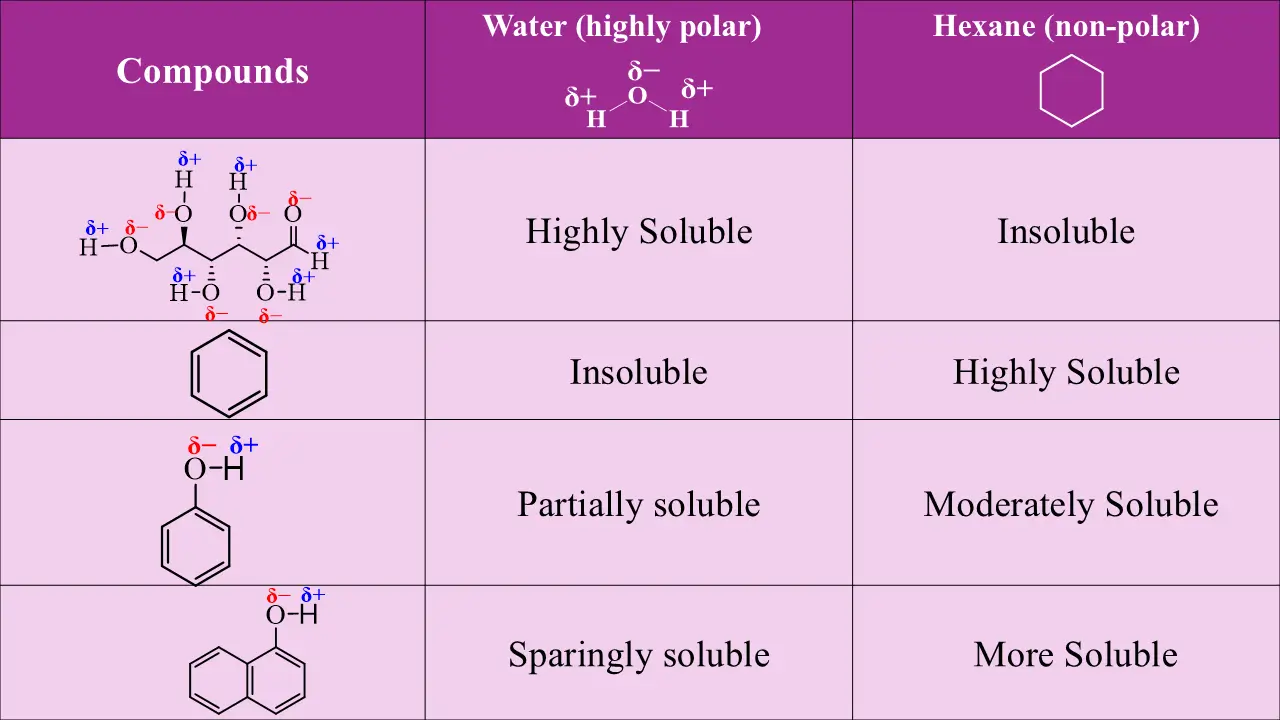
An organic compound is soluble in water if its polar functional group and hydrogen-bonding ability dominate over its hydrocarbon chain; otherwise, it becomes insoluble, but its ionic salts are usually water-soluble.
General Solubility Criteria of organic Compounds in water
- Short carbon chain → higher solubility
- Long carbon chain → lower solubility
- Aromatic rings reduce solubility
- Hydrogen bonding increases solubility but has limits
- Ionic salts are usually soluble in water
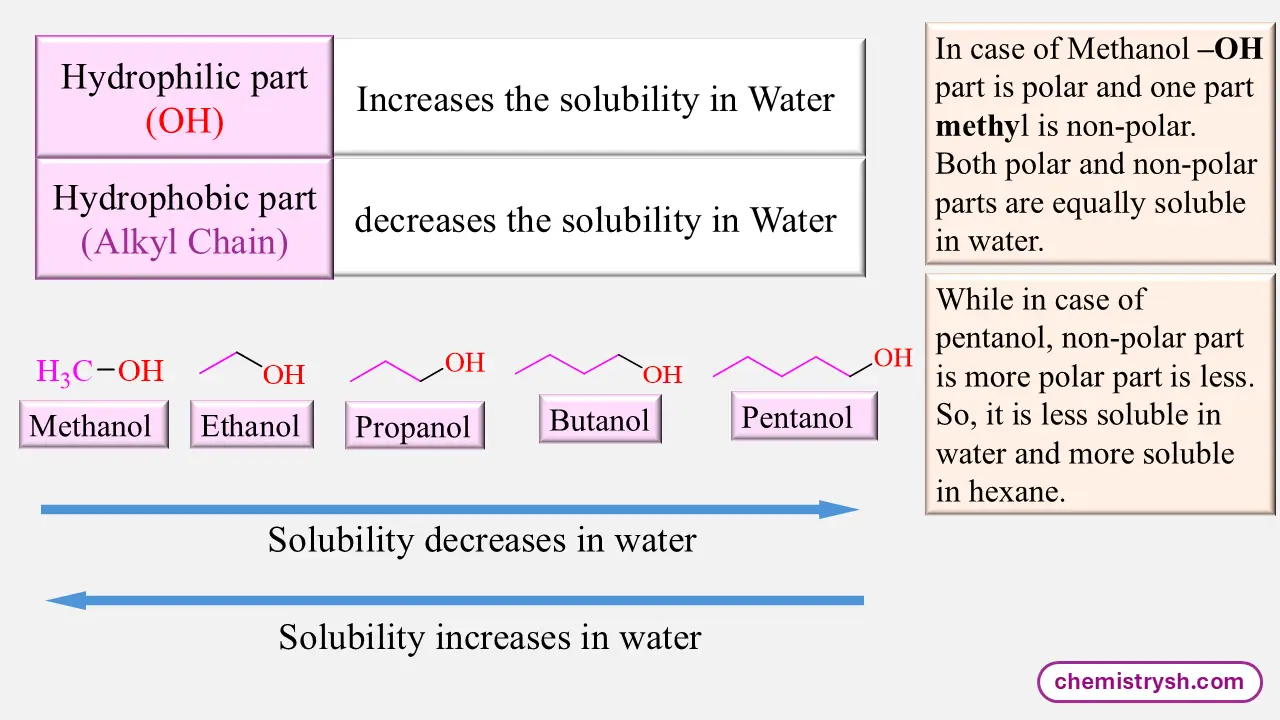
Effect of Carbon Chain length in Alcohols
As the number of carbon atoms increases, the hydrophobic character of the molecule increases and water solubility decreases.
Key Rule:
Solubility decreases as carbon chain length increases.
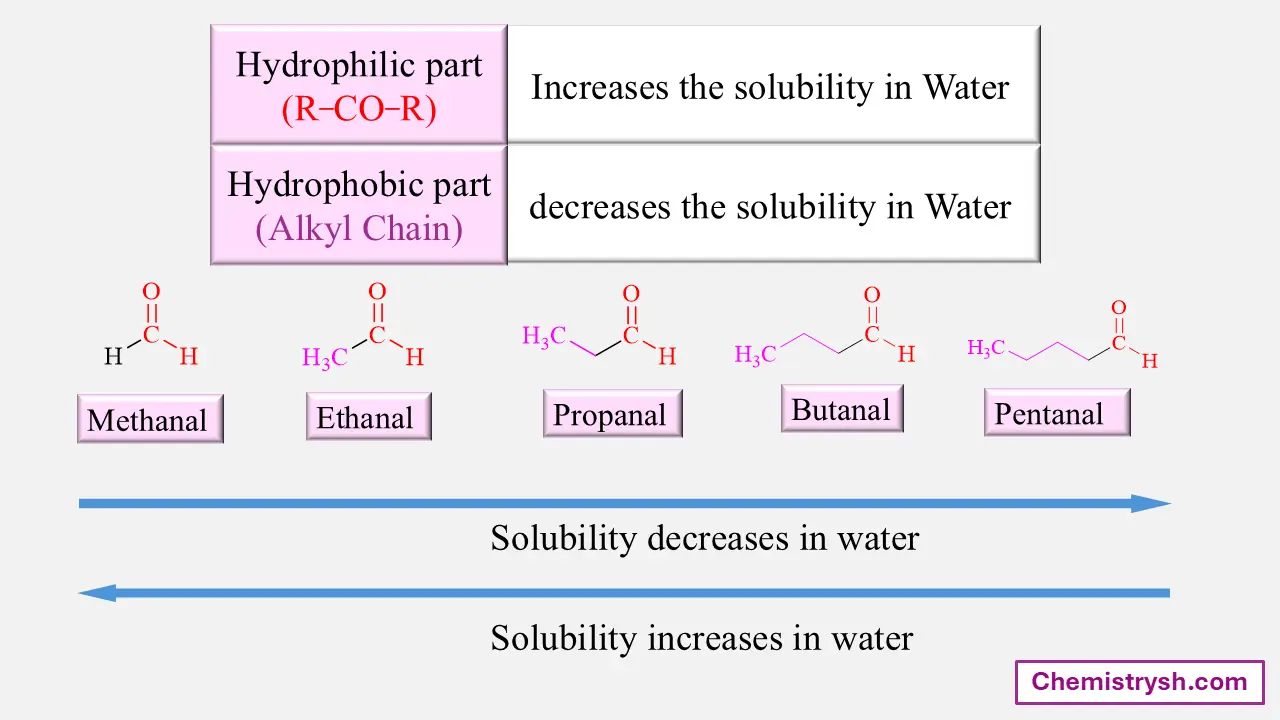
Effect of Carbon Chain Length in Carbonyls
As the number of carbon atoms increases, the hydrophobic character of the molecule increases and water solubility decreases.
Key Rule:
Solubility decreases as carbon chain length increases.
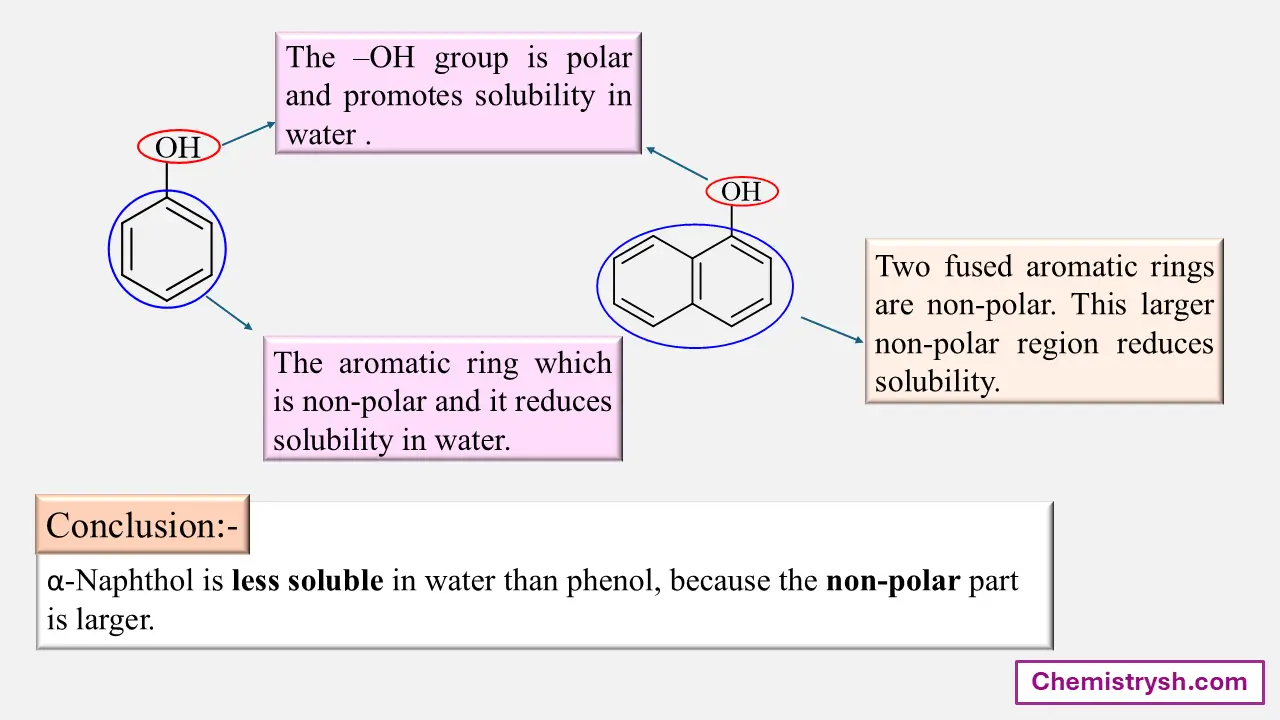
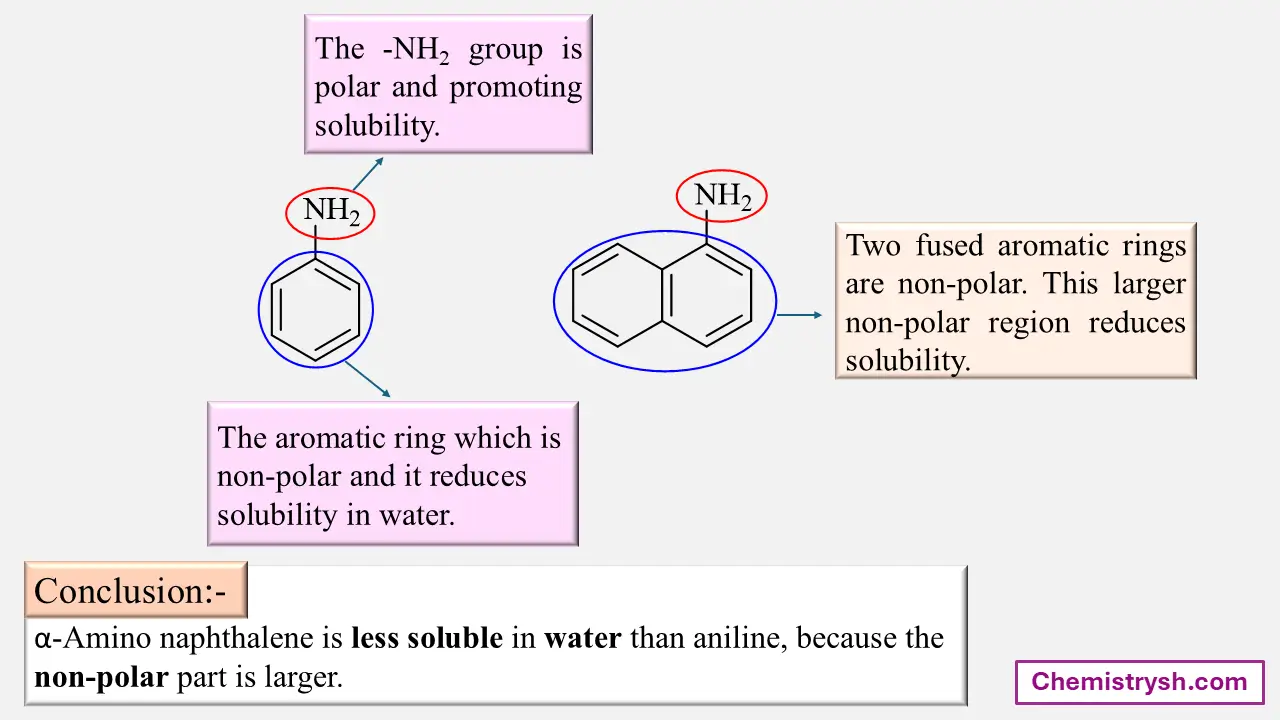
2. Aromatic Ring Effect
Aromatic rings significantly reduce solubility by increasing hydrophobic character.
Phenol is partially soluble because the –OH group adds polarity. when number of -OH groups attached to benzene increases, solubility increases. In this case, the non-polar benzene ring is constant, and adding –OH groups, increases polarity, which increases its solubility in water.
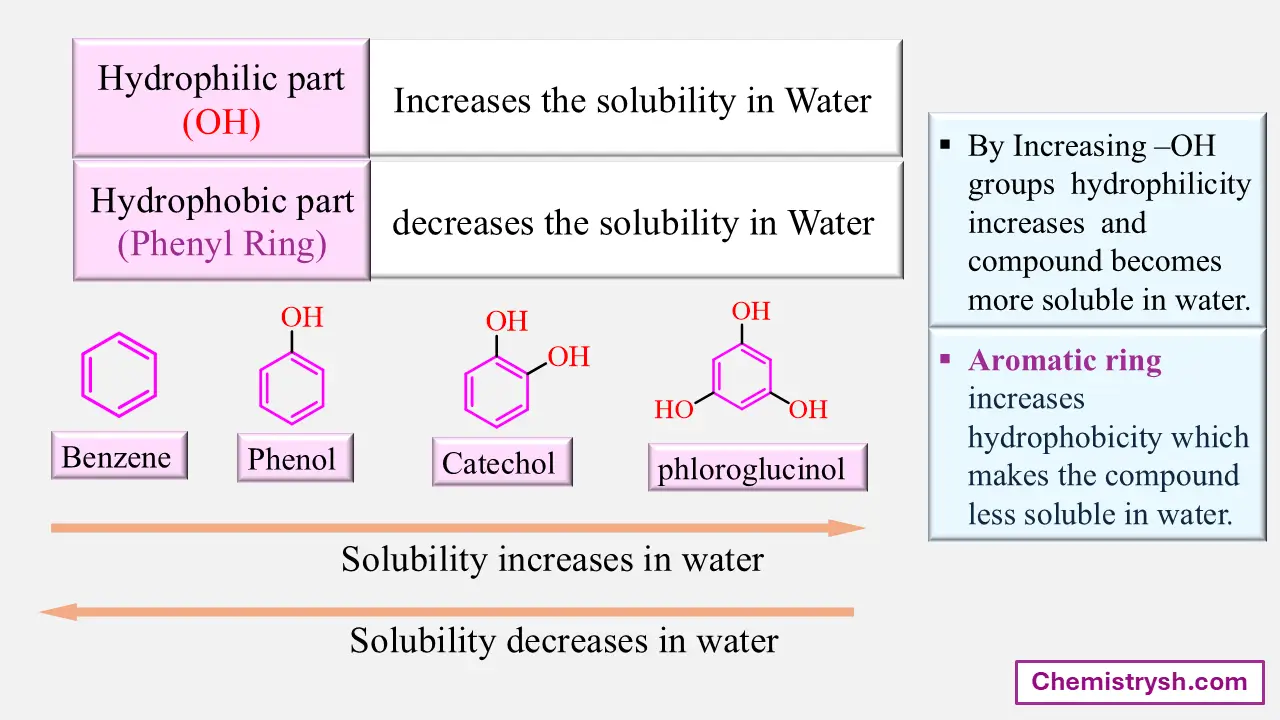
Effect of Polarity and Hydrogen Bonding
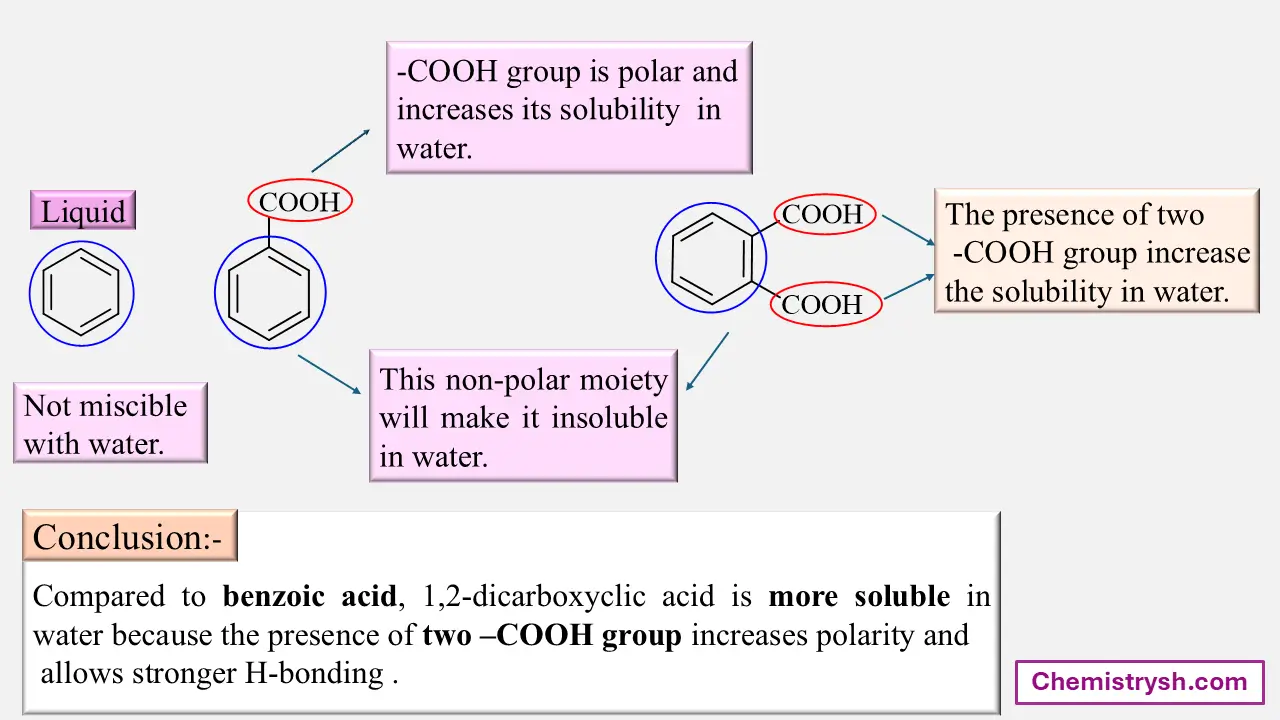
Organic compounds that contain polar functional groups such as –OH, –COOH, –NH₂, or –CONH₂ can form hydrogen bonds with water. This interaction promotes solubility.
Example:
Methanol is soluble due to strong hydrogen bonding and a short carbon chain, whereas hexanol is insoluble because the long hydrocarbon chain suppresses polarity.
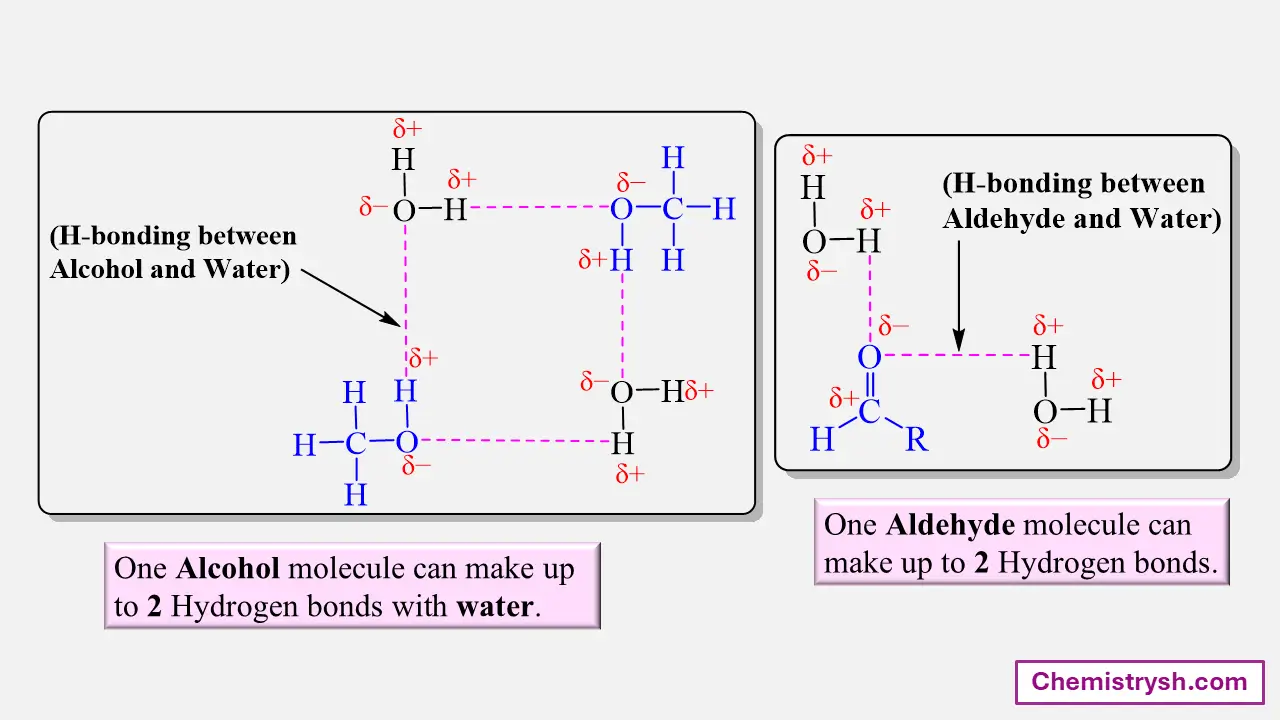
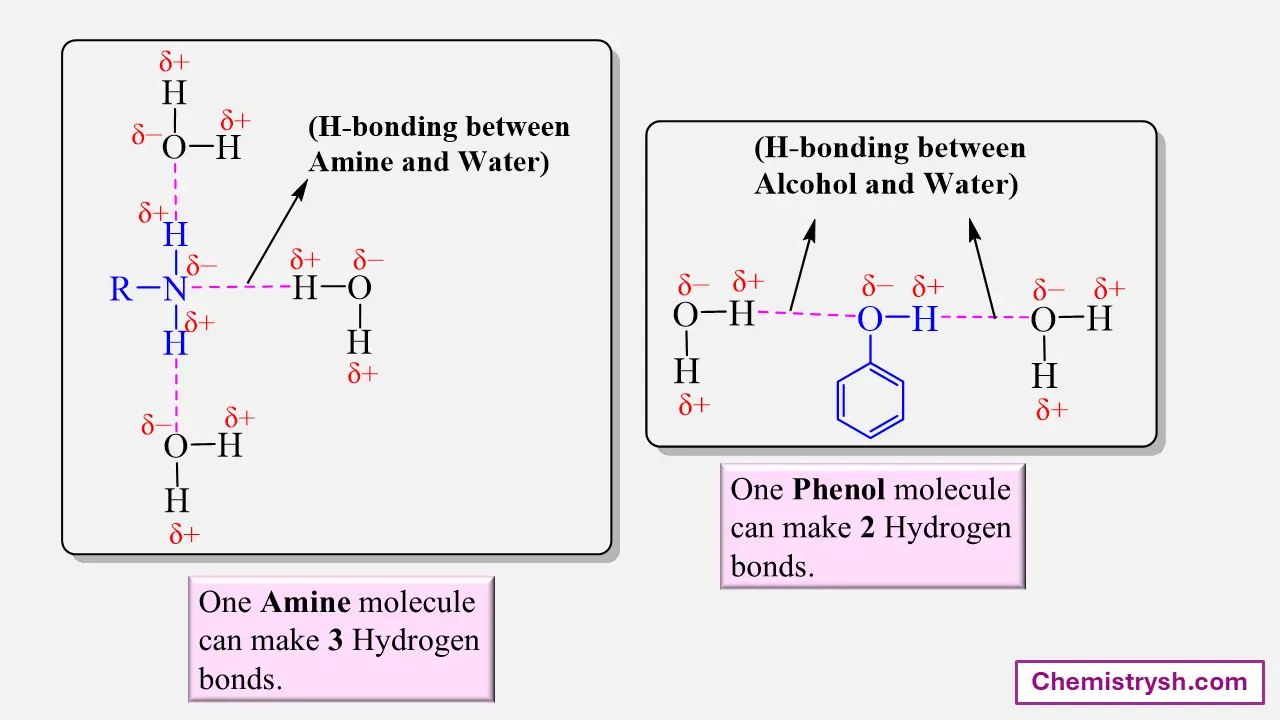
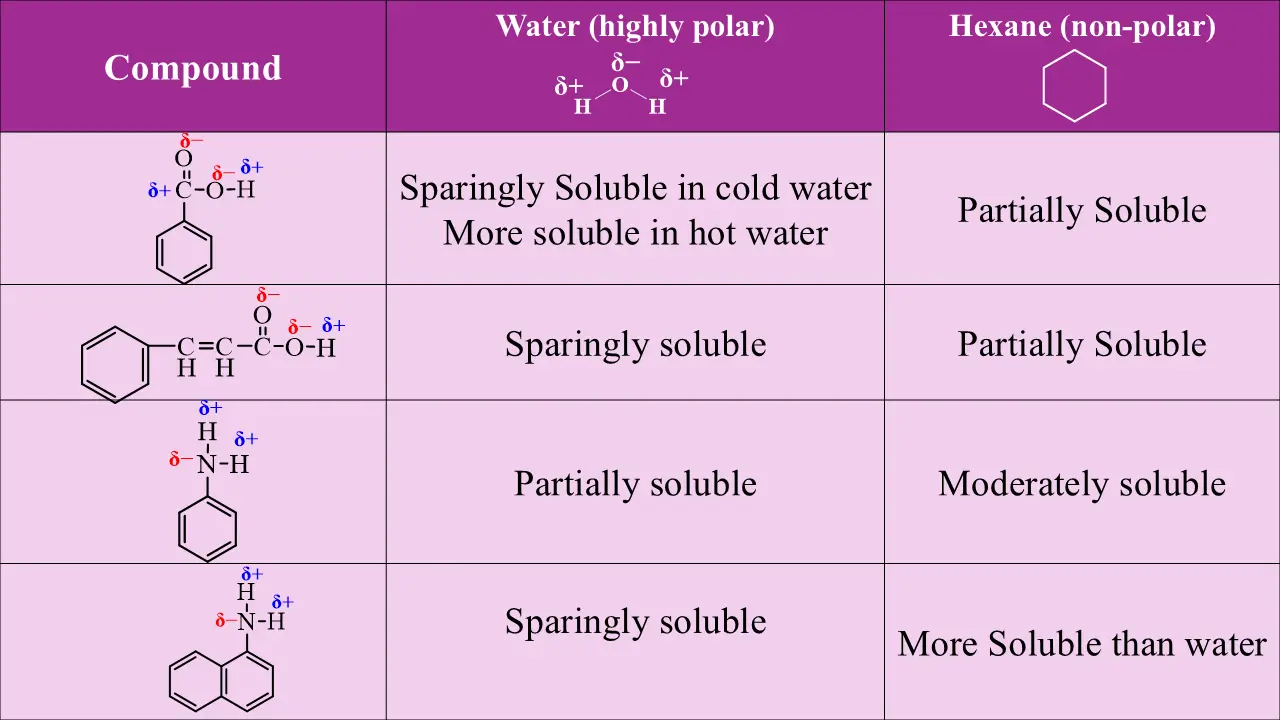
Ionization and Salt Formation
Non-ionized organic compounds may be poorly soluble. When converted into ionic salts, solubility increases sharply due to strong ion–dipole interactions with water. All compounds of sodium (Na⁺), potassium (K⁺), and ammonium (NH₄⁺) are highly soluble in water due to the strong interaction of these ions with water molecules.
Key Principle:
Salts of organic acids and bases are generally soluble in water.
Benzoic acid is slightly soluble in water due to limited hydrogen bonding of its –COOH group. In contrast, sodium benzoate is highly soluble because the ionic –COO⁻Na⁺ group interacts strongly with water.
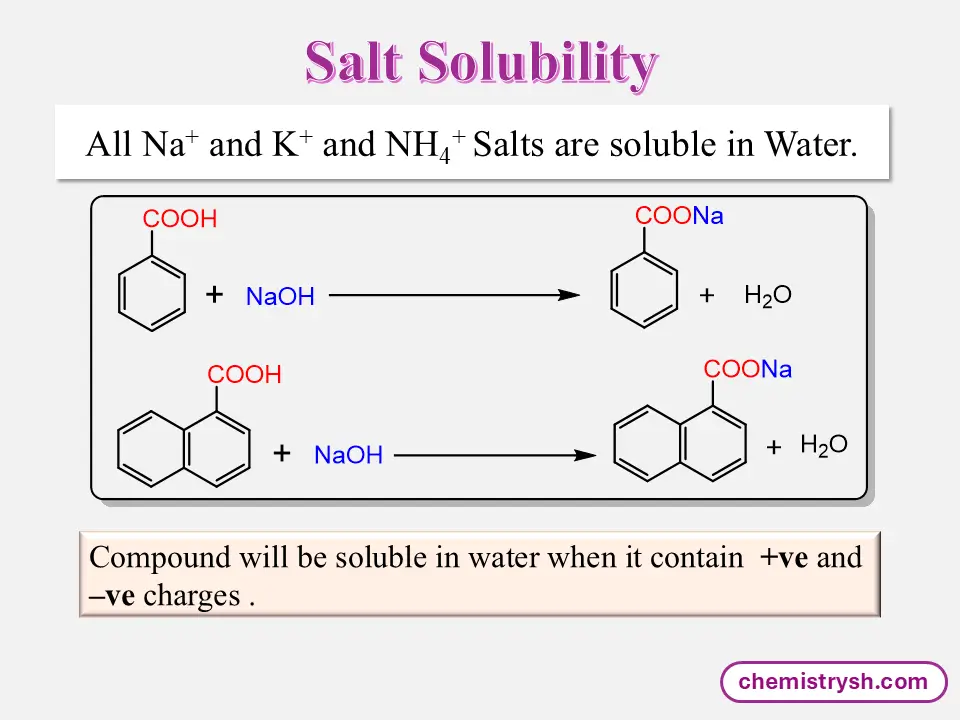
Ionization and Salt Formation
Phenol is moderately soluble in water due to its –OH group forming hydrogen bonds, while sodium phenoxide is completely soluble because the ionic –O⁻Na⁺ group interacts strongly with water, enhancing solubility.
Aniline is slightly soluble in water because the –NH₂ group forms limited hydrogen bonds. In contrast, anilinium chloride is readily soluble due to the ionic –NH₃⁺Cl⁻ group interacting strongly with water.
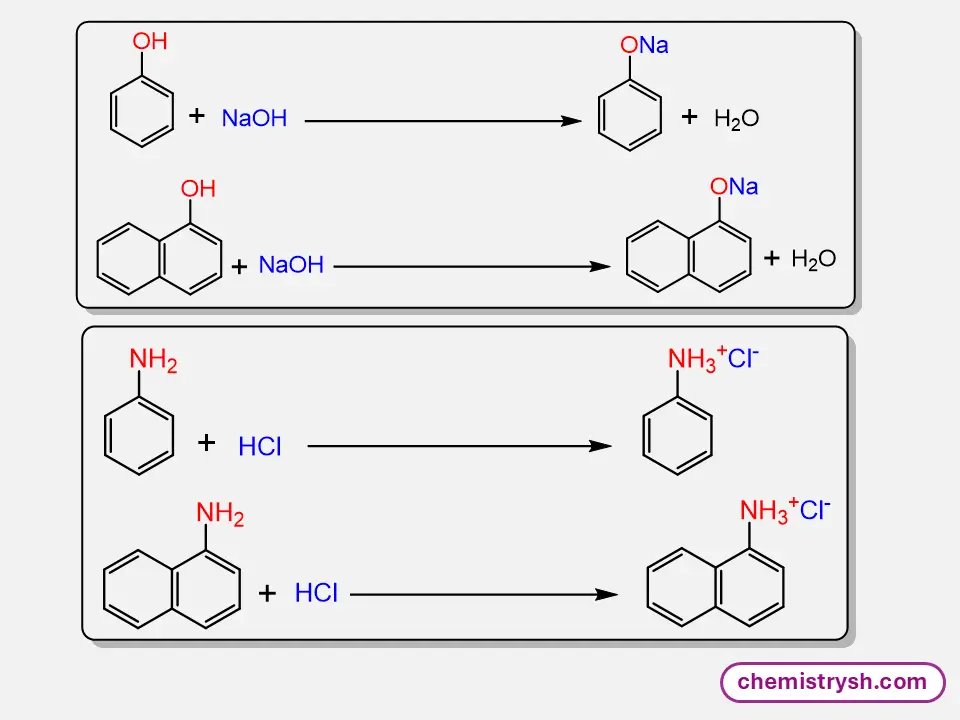
Nature of the Functional Group
Different functional groups influence solubility differently.
“Like Dissolves Like”
Water dissolves polar substances, and hexane dissolve none polar compounds.
Chemistry Terms Related to Solubility, Polarity, and Intermolecular Forces
|
Terms |
Explanation |
Examples |
|---|---|---|
|
Polar |
Having Partial +ve an –ve charges |
Water, Ethanol |
|
Hydrophilic |
Having affinity towards water |
Sugars (glucose), Alcohols |
|
Hydrophobic |
Having no affinity towards water |
Alkanes , Oils and fats |
|
Soluble |
Substances which dissolve in water |
Sugars |
|
Sparingly soluble |
Only a very small amount dissolves |
Benzophenone |
|
Insoluble |
Does not dissolve in water |
Benzene, Hexane |
|
Miscible |
Two liquids that mix completely |
Alcohol + Water |
|
Immiscible |
Two liquids that don’t mix with each other |
Hexane + Water |
|
Partially soluble |
More than sparingly soluble |
Ether |
|
Like dissolve like |
Polar solute dissolves in polar solvent Non polar solutes dissolves in non-polar solvents |
Sugar in Water (polar) Grease (Hydrocarbon) in Hexane (non-polar) |
|
Hydrogen Bonding |
Specific type of interaction between hydrogen atom and electronegative atom (like F, O and N) |
Water and Ammonia |
|
London Dispersion Forces |
Very weak intermolecular forces between non-polar molecules |
Hexane Benzene |
Multiple Choice Questions
MCQ 1
1. Which factor primarily decreases the solubility of alcohols in water as the series increases?
A. Increase in hydrogen bonding
B. Increase in polarity
C. Increase in alkyl chain length
D. Presence of hydroxyl group
MCQ 2
2. Which alcohol is most soluble in water?
A. Pentanol
B. Butanol
C. Propanol
D. Methanol
MCQ 3
3. Why is phenol only slightly soluble in water despite having an –OH group?
A. Absence of hydrogen bonding
B. Presence of aromatic ring
C. Low molecular weight
D. High polarity
MCQ 4
4. Which statement correctly explains the effect of carbon chain length on solubility?
A. Longer chains increase solubility in water
B. Chain length has no effect on solubility
C. Longer chains increase hydrophobic character
D. Short chains decrease hydrogen bonding
MCQ 5
5. Which compound becomes highly soluble in water after conversion to its salt?
A. Phenol
B. Ethanol
C. Benzoic acid
D. Hexane
MCQ 6
6. Why are ionic salts of organic acids and bases generally soluble in water?
A. Due to covalent bonding
B. Due to ion–dipole interactions
C. Due to aromaticity
D. Due to weak polarity
FAQ’s
References
- Vogel, A. I., Furniss, B. S., Hannaford, A. J., Smith, P. W. G., & Tatchell, A. R. (1989). Vogel’s Textbook of Practical Organic Chemistry (5th ed.). Longman / Wiley.
- Pavia, D. L., Lampman, G. M., Kriz, G. S., & Engel, R. G. (2016). A Small-Scale Approach to Organic Laboratory Techniques (3rd ed.). Cengage Learning.
- Atkins, P., de Paula, J., & Keeler, J. (2018). Atkins’ Physical Chemistry (11th ed.). Oxford University Press.
- Clayden, J., Greeves, N., Warren, S., & Wothers, P. (2012). Organic Chemistry (2nd ed.). Oxford University Press.
- Solomons, T. W. G., & Fryhle, C. B. (2011). Organic Chemistry (11th ed.). Wiley.
- Mann, F. G., & Saunders, B. C. (1990). Practical Organic Chemistry (3rd ed.). Pearson Education.