The Tollens test, also known as the silver mirror test, is a qualitative chemical test used to identify aldehydes. In this test, an aldehyde reduces Tollens reagent to metallic silver, which deposits as a bright silver mirror inside the test tube. The test is widely used in organic chemistry to distinguish aldehydes from ketones.
The silver mirror test works on the principle that aldehydes are easily oxidized, whereas ketones generally resist oxidation under mild conditions.
Named after German chemist Bernhard Tollens who developed it in 1882, the test produces a characteristic silver mirror coating on the inside of the test tube when a positive result occurs.
This test also detects certain reducing sugars like glucose, making it useful in biochemistry. The reaction involves the diammine silver(I) complex, which reduces to metallic silver, forming the mirror. Its selectivity, visual clarity, and educational value make it a key experiment for distinguishing aldehydes from ketones and studying carbonyl chemistry under mild conditions.

Tollens Reagent Formula, Structure and Preparation
Tollens reagent is a freshly prepared ammoniacal solution of silver nitrate containing the diamminesilver(I) complex. It acts as a mild oxidizing agent and is responsible for producing the characteristic silver mirror during the Tollens test.
Tollens Reagent Structure and Formula
The Tollens reagent structure consists of a diammine silver(I) complex with the chemical formula [Ag(NH₃)₂]⁺. This cationic complex forms when silver ions coordinate with ammonia molecules in an aqueous solution. The silver ion in this complex exists in the +1 oxidation state, making it a mild oxidizing agent capable of oxidizing aldehydes.
The structure can be represented as:
[Ag(NH₃)₂]OH or [Ag(NH₃)₂]⁺ + OH⁻
In this complex, the silver ion is surrounded by two ammonia molecules in a linear geometry. The presence of hydroxide ions in the solution maintains the basic pH necessary for the test.
Preparation of Tollens Reagent
The preparation of Tollens reagent requires careful attention to detail and must be done immediately before use. Here’s the detailed process for making Tollens reagent:
Step 1: Dissolve silver nitrate (AgNO₃) in distilled water to create a dilute aqueous solution (typically 2-3% solution).
Step 2: Add dilute sodium hydroxide (NaOH) solution dropwise to the silver nitrate solution. A brown precipitate of silver oxide (Ag₂O) forms initially.
Step 3: Add dilute ammonia solution (NH₃) dropwise while stirring continuously. The brown precipitate gradually dissolves as the diammine silver(I) complex forms, resulting in a clear, colorless solution.
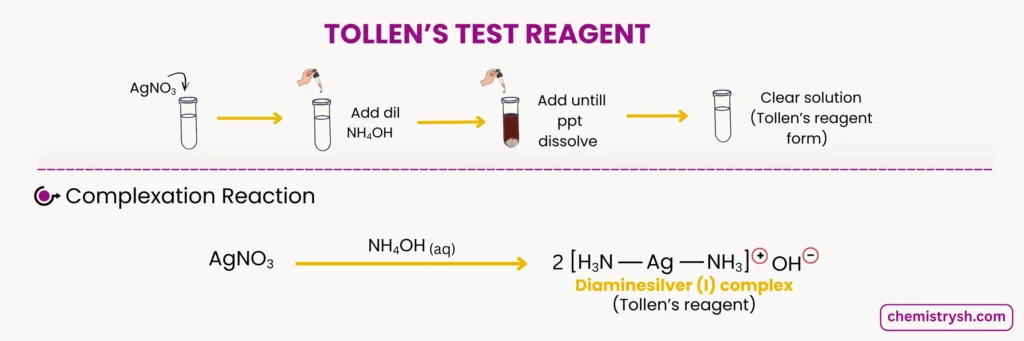
Important Notes on Tollens Reagent Preparation:
- The reagent must be used immediately after preparation
- Never store Tollens reagent, as it can form explosive silver compounds upon standing
- Always prepare only the amount needed for immediate testing
- The solution should be clear and colorless when properly prepared
- Maintain slight excess of ammonia to keep the complex stable
Principle of Tollens’ Test
The Tollens Test works because aldehydes are oxidized to carboxylic acids, while silver ions are reduced to metallic silver. The formation of a silver mirror indicates a positive test. Ketones generally do not react under these conditions.
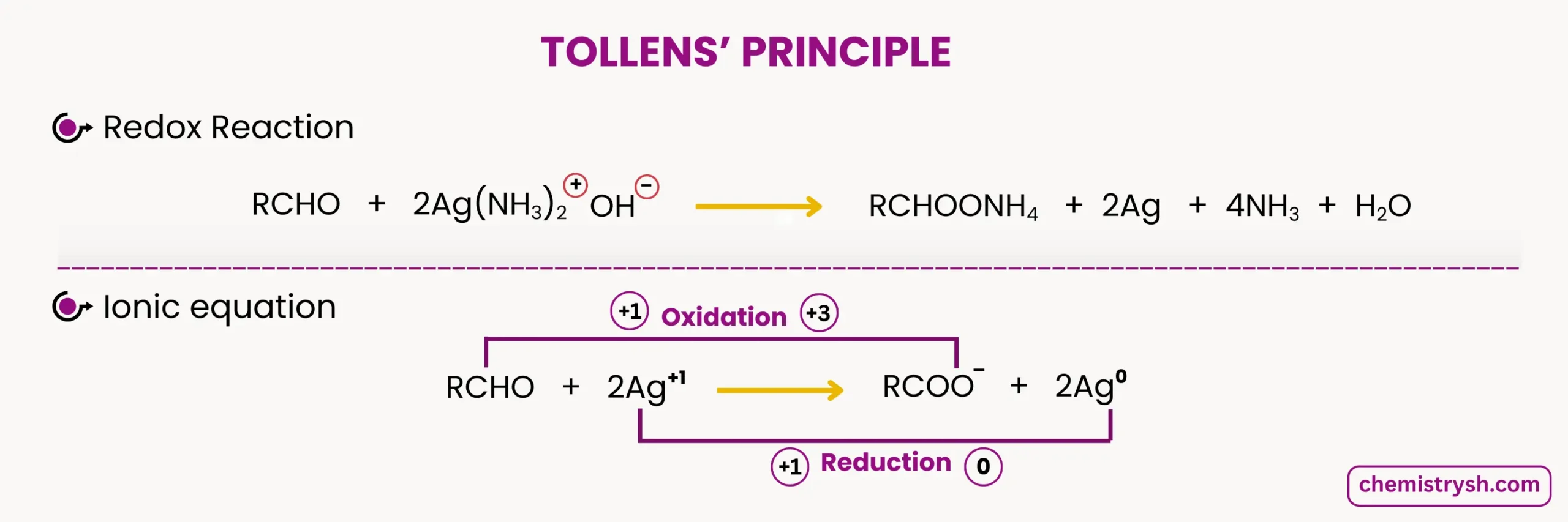
Silver Mirror (Tollens) Test Reaction and Equation
The silver mirror test reaction is an oxidation–reduction reaction. The aldehyde is oxidized, while silver ions are reduced to metallic silver.
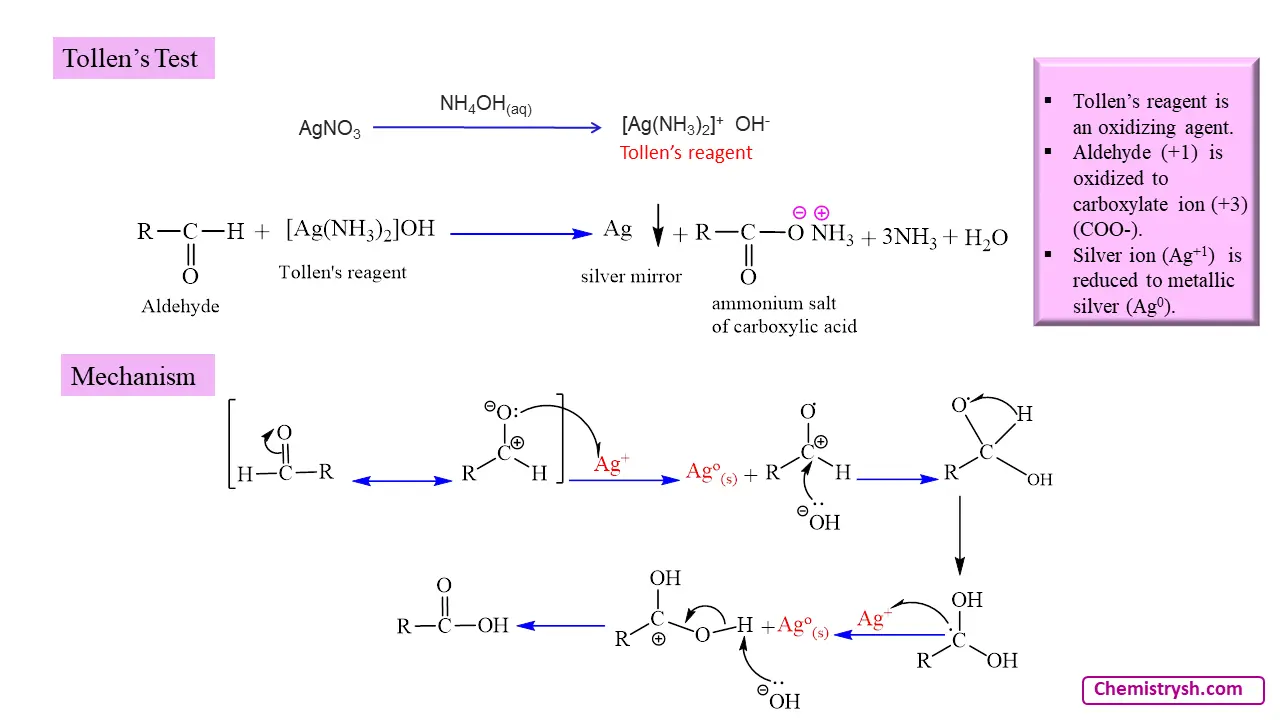
Mechanism of Tollens Test (Silver Mirror Test Mechanism)
The mechanism of Tollens test involves following steps:
- Reduction of Ag⁺ ions to metallic silver (Ag⁰), which deposits as a mirror.
- Formation of the diamminesilver(I) complex from silver nitrate and ammonia.
- Oxidation of the aldehyde group to a carboxylate ion.
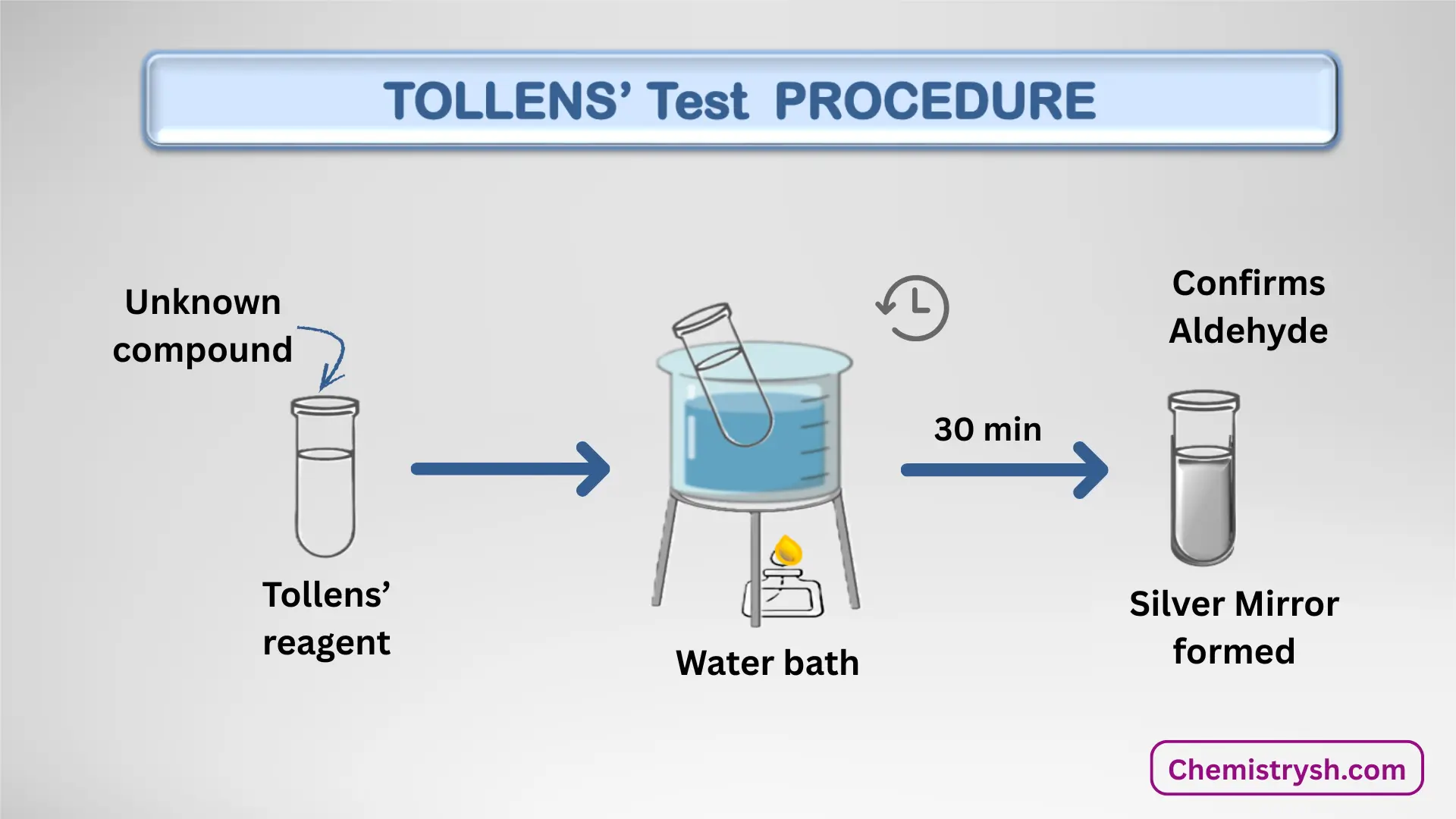
Procedure of Tollens’ Test
The silver mirror (tollens) test procedure involves the following steps:
- Take a clean test tube.
- Add 2–3 mL of Tollens reagent.
- Add a few drops of the sample solution.
- warm in a water bath for 30 minutes
- Observe the test tube walls for silver coating.
If aldehyde is present, a silver mirror in the test tube appears.
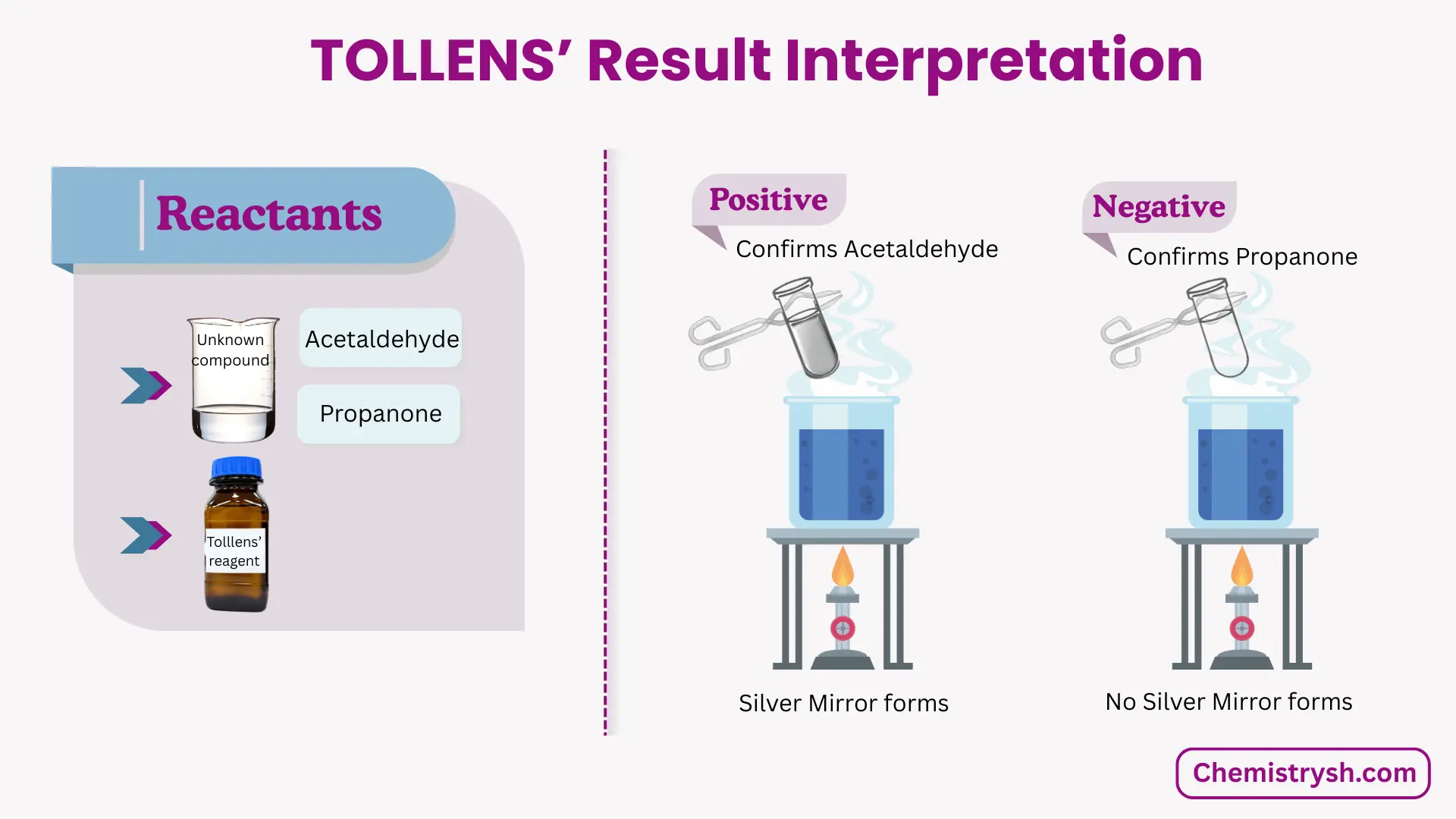
Tollens Test Results – Positive and Negative
Positive Tollens Test
A positive Tollens test result is indicated by the formation of a bright silver mirror or grey precipitate of silver. This confirms the presence of an aldehyde.
Alpha-hydroxy ketones also give positive tollens test as a special case.
Negative Tollens Test
A negative Tollens test shows no silver mirror formation, indicating the absence of aldehydes.
For example, phenolic compounds do not respond to the Tollens reagent but can be easily identified using the Ferric chloride test, which produces a characteristic color change. Using both tests together improves accuracy during functional group identification and avoids false conclusions.
Tollens Test for Aldehydes
The Tollens test for aldehydes is based on the fact that aldehydes are easily oxidized due to the presence of an aldehydic hydrogen attached to the carbonyl carbon. This structural feature makes the carbonyl carbon more susceptible to oxidation under mild conditions.
When an aldehyde reacts with Tollens reagent, it is oxidized to a carboxylate ion, while silver ions are reduced to metallic silver, producing the characteristic silver mirror test for aldehyde, also known as the aldehyde silver mirror test.
For example, when Tollens reagent with benzaldehyde is gently warmed, benzaldehyde is oxidized to benzoate ions, resulting in the formation of a bright silver mirror inside the test tube.
Compounds That Do Not Give Tollens Test
Most ketones and alcohols do not reduce Tollens reagent and therefore give a negative Tollens test. This property makes the test useful for distinguishing aldehydes from ketones.
Uses and Applications of Tollens’ Test
- This test is widely used in organic synthesis labs to confirm aldehyde formation and differentiate them from ketones.
- The food industry uses it to test for reducing sugars like glucose and fructose.
- It also serves as a demonstration in chemistry education, demonstrating redox reactions visually.
- It is employed in mirror coating and silver-plating processes.
Conclusion
The Tollens’ test is a simple, reliable, and visual method for detecting aldehydes. It gives immediate results and is widely applicable in labs, industries, and teaching. Its selective reaction with aldehydes makes it a standard tool in organic chemistry.
Multiple Choice Questions
MCQ 1
1. What does the Tollens Test detect?
A. Ketones
B. Alcohols
C. Aldehydes
D. Carboxylic acids
MCQ 2
2. What forms on a positive Tollens Test?
A. Precipitate of copper
B. Silver mirror
C. Gas bubbles
D. Red solution
MCQ 3
3. Does acetone give a positive Tollens Test?
A. No
B. Yes
C. Sometimes
D. Only on heating
MCQ 4
4. Which reagent is used in the Tollens Test?
A. Fehling solution
B. [Ag(NH₃)₂]⁺
C. Benedict’s solution
D. KMnO₄
MCQ 5
5. Can alpha-hydroxy ketones give a positive result?
A. Yes
B. No
C. Only in Fehling Test
D. Only in acidic solution
MCQ 6
6. The silver mirror observed in Tollen’s test is due to the formation of:
A. Silver chloride
B. Silver oxide
C. Metallic silver
D. Silver carbonate
Viva questions
FAQ’s
References of Tollens’ test
- Vogel, A. I. (1996). Vogel’s Textbook of Practical Organic Chemistry (5th ed.). Longman Scientific & Technical.
- Morrison, R. T., & Boyd, R. N. (1992). Organic Chemistry (6th ed.). Prentice Hall.
- Finar, I. L. (2006). Organic Chemistry: Practical Workbook (6th ed.). Pearson Education.
- Bansal, R. K. (2010). A Textbook of Organic Chemistry (4th ed.). Wiley Eastern Ltd.