The bromine water test is a widely used qualitative test in organic chemistry for detecting unsaturation and reactive aromatic compounds in organic substances.
Test for alkenes and alkynes:
The reddish-brown color of bromine water disappears, indicating the presence of double or triple bonds.
Test for aromatic compounds (aniline or phenol):
The color disappears and a white precipitate appear

What is bromine water
Bromine water is a 2% aqueous solution of bromine. It has areddish-brown color, reacts with unsaturated compounds (alkenes/alkynes) and some reactive aromatic compounds (phenols/anilines).
Prepration
Bromine water is prepared by adding 1 ml of liquid bromine to about 50 mL of distilled water in a clean flask and shaking gently until a light brown solution is formed.
Bromine in CCl₄ is prepared by adding 1 ml of liquid bromine to about 50 mL of carbon tetrachloride in a dry bottle and shaking gently to obtain a reddish-brown solution. Both solutions should be properly labeled and handled with care.
Bromine is a highly reactive, toxic, and corrosive halogen with strong oxidizing properties (So it should be handeled with care and in fume hood.)
Point to think
Non-polar bromine dissolves in polar water to form bromine water how?
It reacts with water to form HBr and HOBr, creating equillibrium that continually pulls more Br₂ into solution.
Tid Bits
- Bromine is the only non-metal (halogen) element that is naturally in a liquid state at standard room temperature and pressure.
- It’s brown-red color appears because bromine’s electrons absorb certain wavelengths of visible light and move to higher energy levels, leaving the complementary brown/red wavelengths reflected back to our eyes.

Principle of bromine water
1. Alkene
- Undergo electrophilic addition with bromine.
- Bromine adds across the double bond.
- The reddish-brown color of bromine water disappears completely.
2. Alkyne
- React slower than alkenes.
- Partial electrophilic addition occurs.
- Color fades but may not disappear completely due to lower reactivity.
3. Aromati compouds (phenols, anilines)
- Undergo electrophilic substitution with bromine.
- Color disappears and a white precipitate is formed.
Reactions of bromine water
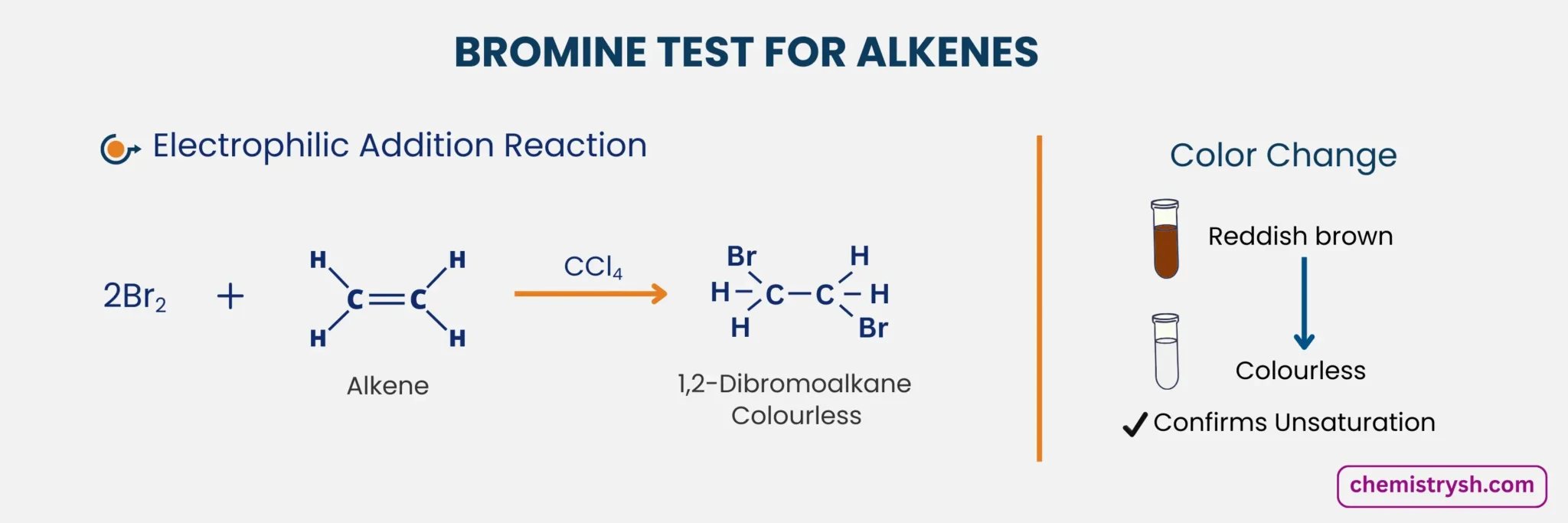
1. Reaction With Alkenes
In this reaction, bromine adds across the carbon–carbon double bond through an electrophilic addition mechanism. When bromine water is added to the unsaturated compound, the reddish-brown color of bromine disappears, indicating that the double bond has reacted.
The product formed is a vicinal dibromo compound, in which the two bromine atoms become attached to adjacent carbon atoms of the former double bond.
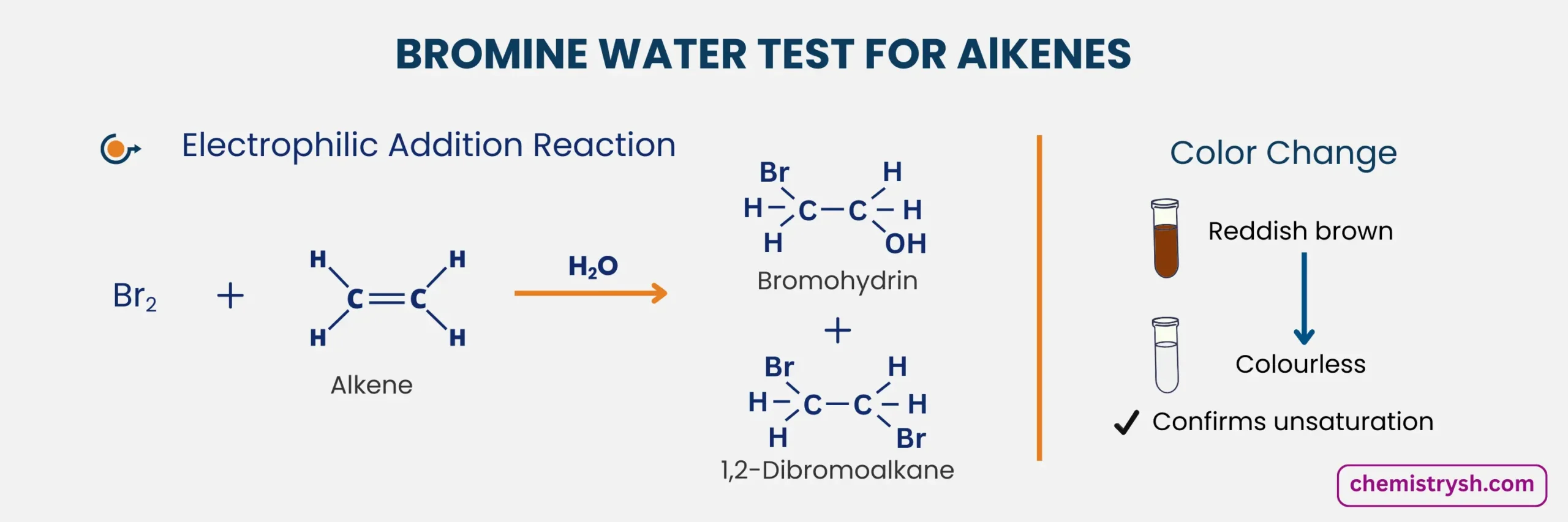
2. Reaction With Alkene in Water
Alkenes react with bromine in water to form a bromohydrin. A bromonium ion forms first, then water opens it, adding –OH to one carbon and Br to the other, giving anti-addition and decolorizing bromine water.
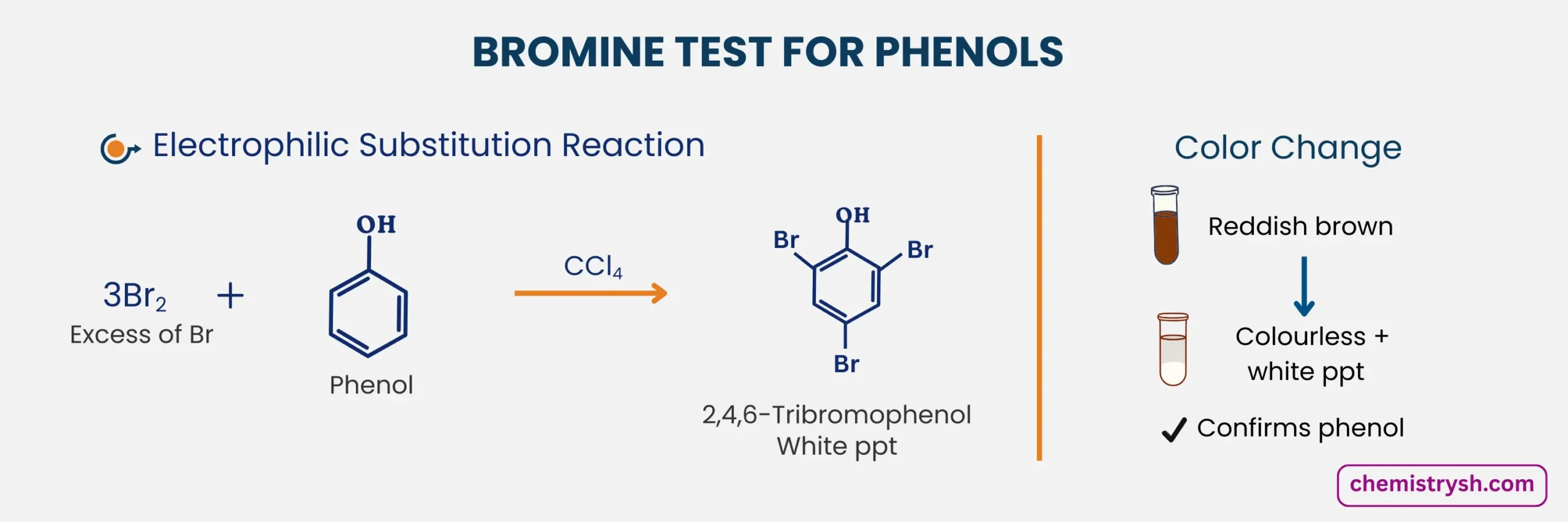
3. reaction With Phenol
Phenol undergoes electrophilic substitution with bromine water at the activated ortho and para positions, causing the color to disappear and forming a white precipitate of 2,4,6-tribromophenol.
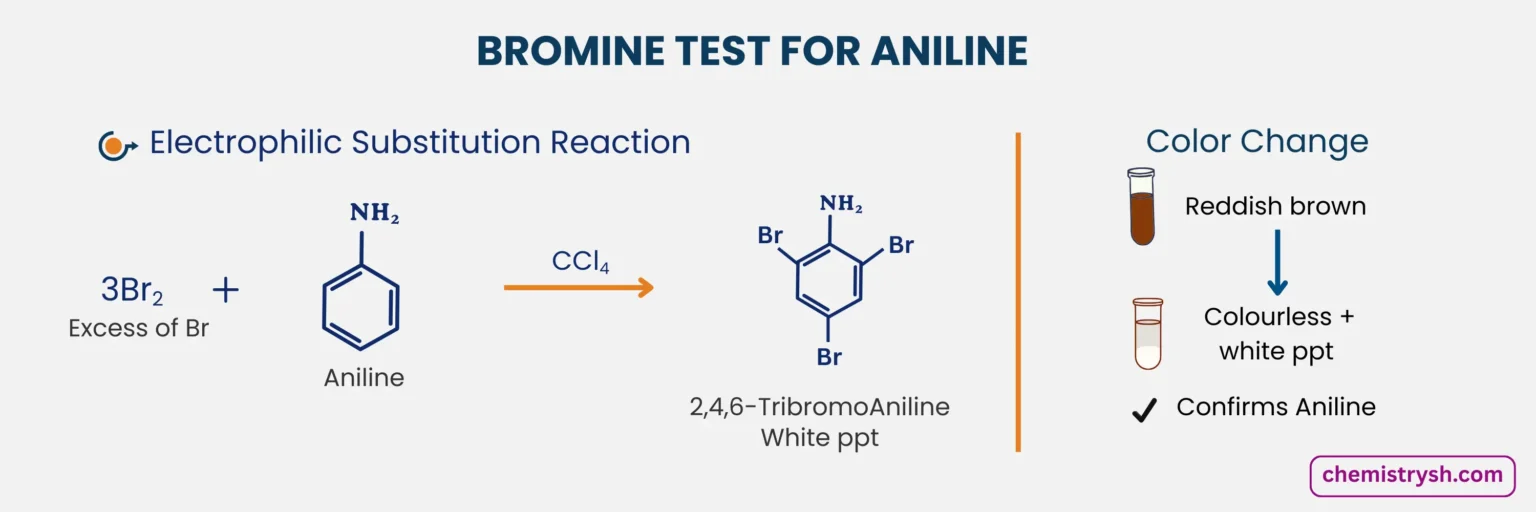
4. Reaction with Aniline
Aniline reacts rapidly with bromine water through electrophilic substitution. The –NH₂ group strongly activates the ring, causing bromine to substitute at the ortho and para positions. The bromine water is decolorized, and a white precipitate of 2,4,6-tribromoaniline forms.
Quick insight box
Why does phenol react with bromine water while benzene does not?
Phenol reacts readily with bromine water because the –OH group activates the aromatic ring via resonance (+R effect), increasing electron density and allowing bromination under mild conditions. Benzene lacks such activation and therefore does not react with bromine water without a catalyst or harsh conditions.
Procedure
(a) For alkenes
- Take 2 ml of the test solution.
- Add 2 to 3 drops of bromine water.
- Shake gently.
- Observe the color change.
- Positive: Color disappears
- Negative: Color remains reddish brown
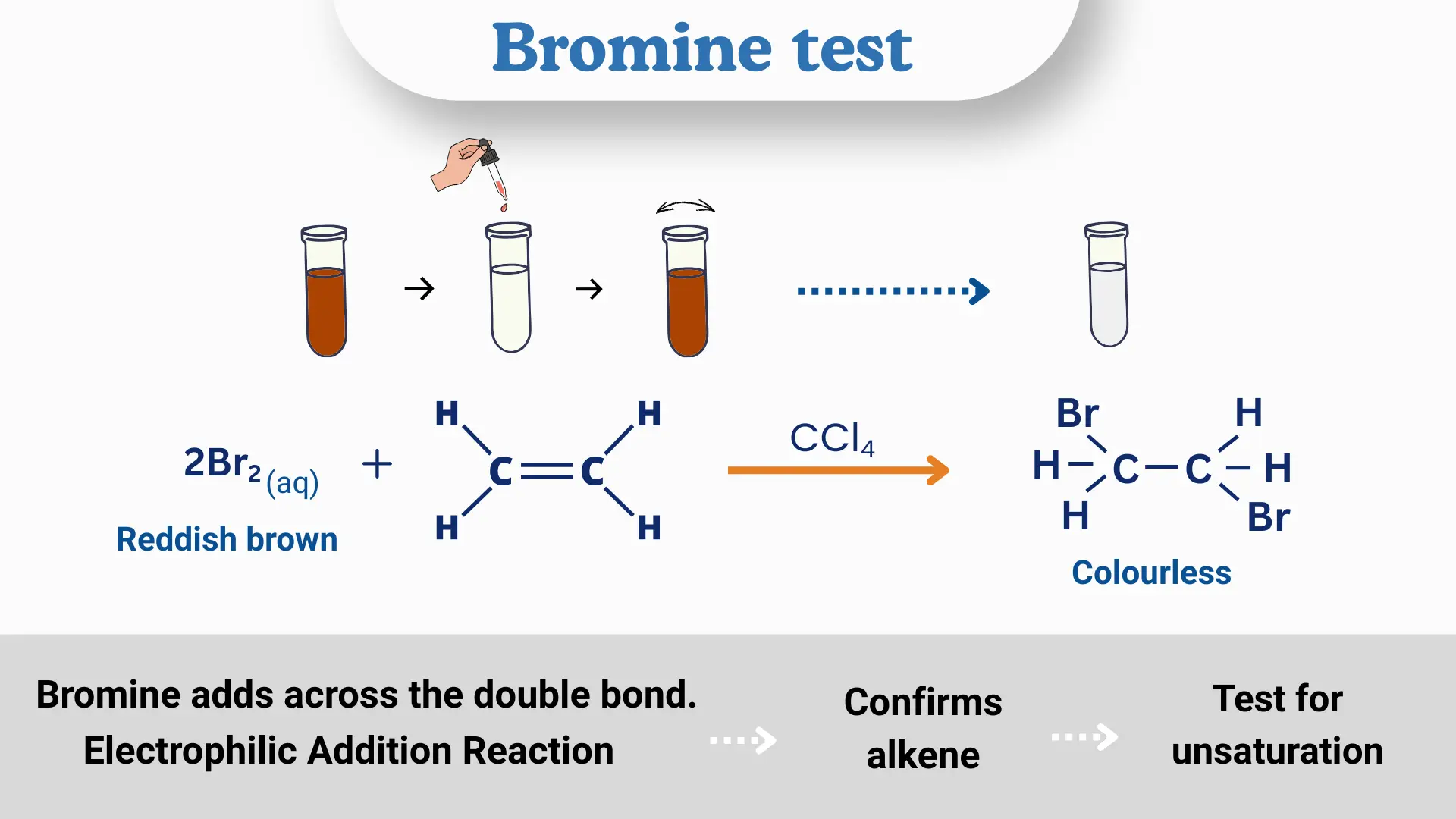
(b) For phenols
- Take 2 ml of the test solution.
- Add 2 to 3 drops of bromine water.
- Shake gently.
- Observe the color change.
- Positive: White precipitate form
- Negative: Color remains reddish brown, indicating the compound is saturated or non reactive.
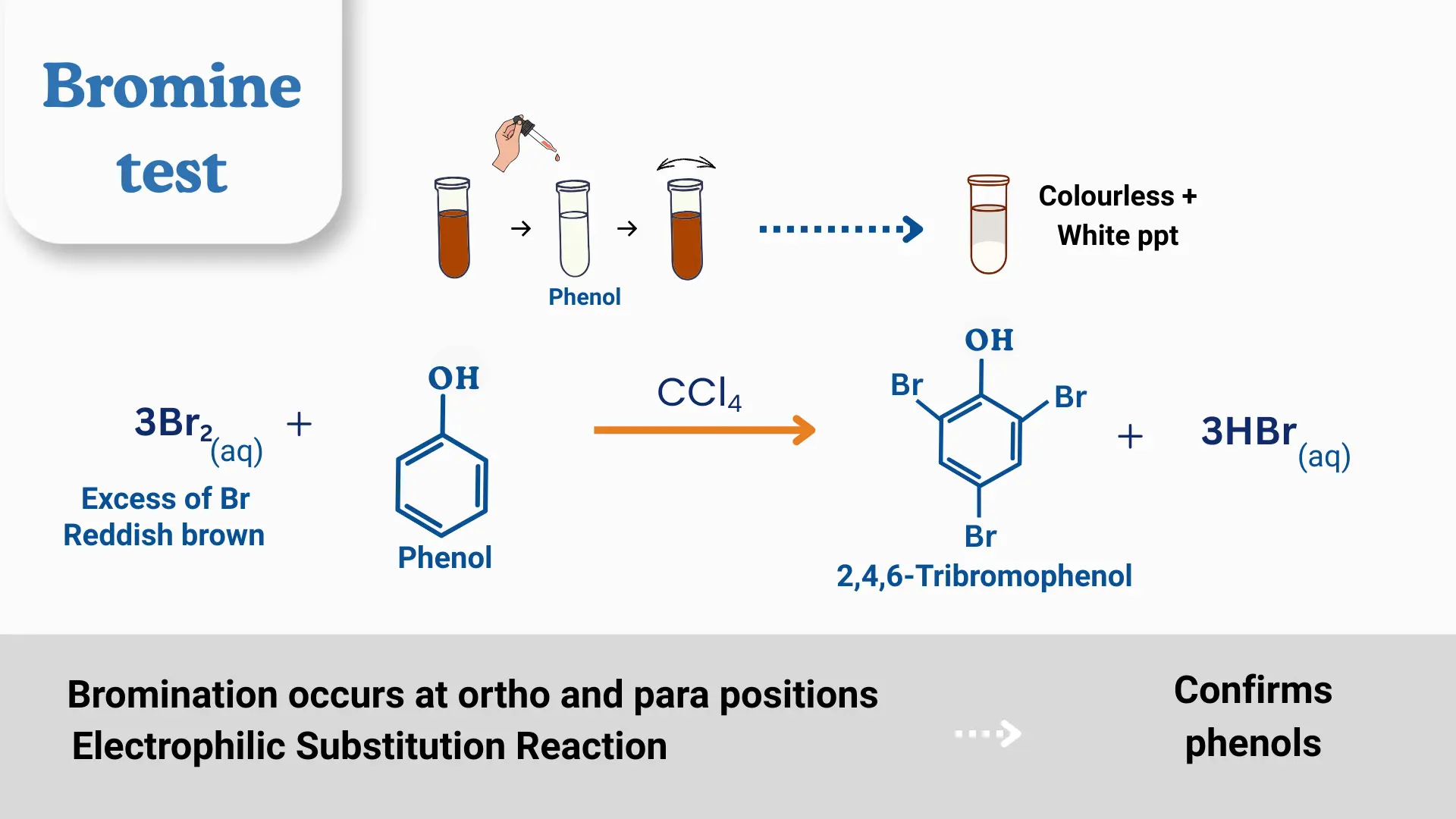
Applications
- Used to detect unsaturation.
- Used to distinguish alkanes from alkenes.
- Used in qualitative organic analysis.
- Used to test phenols and anilines.
- Used in teaching basic organic chemistry reactions.
Conclusion
The bromine water test is a reliable qualitative method for identifying alkenes and phenols based on rapid decolorization or formation of a characteristic precipitate. Its clear visual results, simple procedure, and predictable reaction patterns make it an essential tool for detecting unsaturation and activated aromatic compounds in organic chemistry.
Viva questions
- What is the color of bromine water.
- Why do alkenes decolorize bromine water.
- What product forms when phenol reacts with bromine water.
- Why do alkanes not react with bromine water under normal conditions.
- . What type of reaction takes place in the bromine water test for alkenes.
Multiple Choice Questions
MCQ 1
1. Bromine water is used to test
A. Saturation
B. Unsaturation
C. pH
D. Aromaticity
MCQ 2
2. A positive bromine water test shows_
A. No change
B. Orange to colourless
C. White to brown
D. Purple to pink
MCQ 3
3. Which compound does not react with bromine water?
A. Ethene
B. Phenol
C. Benzene
D. Enol
MCQ 4
4.Phenol with bromine water forms_
A.Blue solution
B. Gas bubbles
C. White precipitate
D. No reaction
MCQ 5
5. Alkanes do not react with bromine water because they are
A. Aromatic
B. Saturated
C. Basic
D. Ionic
MCQ 6
6. The silver mirror observed in Tollen’s test is due to the formation of:
A. Nucleophilic substitution
B. Free radicle substitution
C. Electrophilic addition
D. Nucleophilic addition
FAQs
References of bromine water Test
- Morrison, R. N., & Boyd, R. N. Organic Chemistry, 6th Edition, Pearson, 2010.
- Solomons, T. W. G., & Fryhle, C. B. Organic Chemistry, 12th Edition, John Wiley & Sons, 2017.
- Finar, I. L. Organic Chemistry, Volume 1, 6th Edition, Pearson, 2002.
- O. P. Agarwal A Textbook of Organic Chemistry, 4th Edition, Krishna Prakashan Media, 2010.
- P. L. Soni Practical Organic Chemistry, Sultan Chand & Sons, 2015.
- https://edu.rsc.org/experiments/handling-liquid-bromine-and-preparing-bromine-water/683.article?utm_campaign=share_btn&utm_medium=post&utm_source=navigator


