The Ferric Chloride Test is a simple and widely used qualitative test in organic chemistry, primarily employed to detect phenolic compounds and certain carboxylic acids. This test is based on the ability of ferric ions (Fe³⁺) to form colored complexes when they react with hydroxyl (-OH) groups of phenols or the carboxyl (-COOH) group of some acids.
Phenols react with ferric chloride to give different colors. Aromatic carboxylic acids also react and show various colors. The color depends on the strength of the ligands that form complexes with the Fe³⁺ ion.
The Ferric Chloride Test is given by
Principle
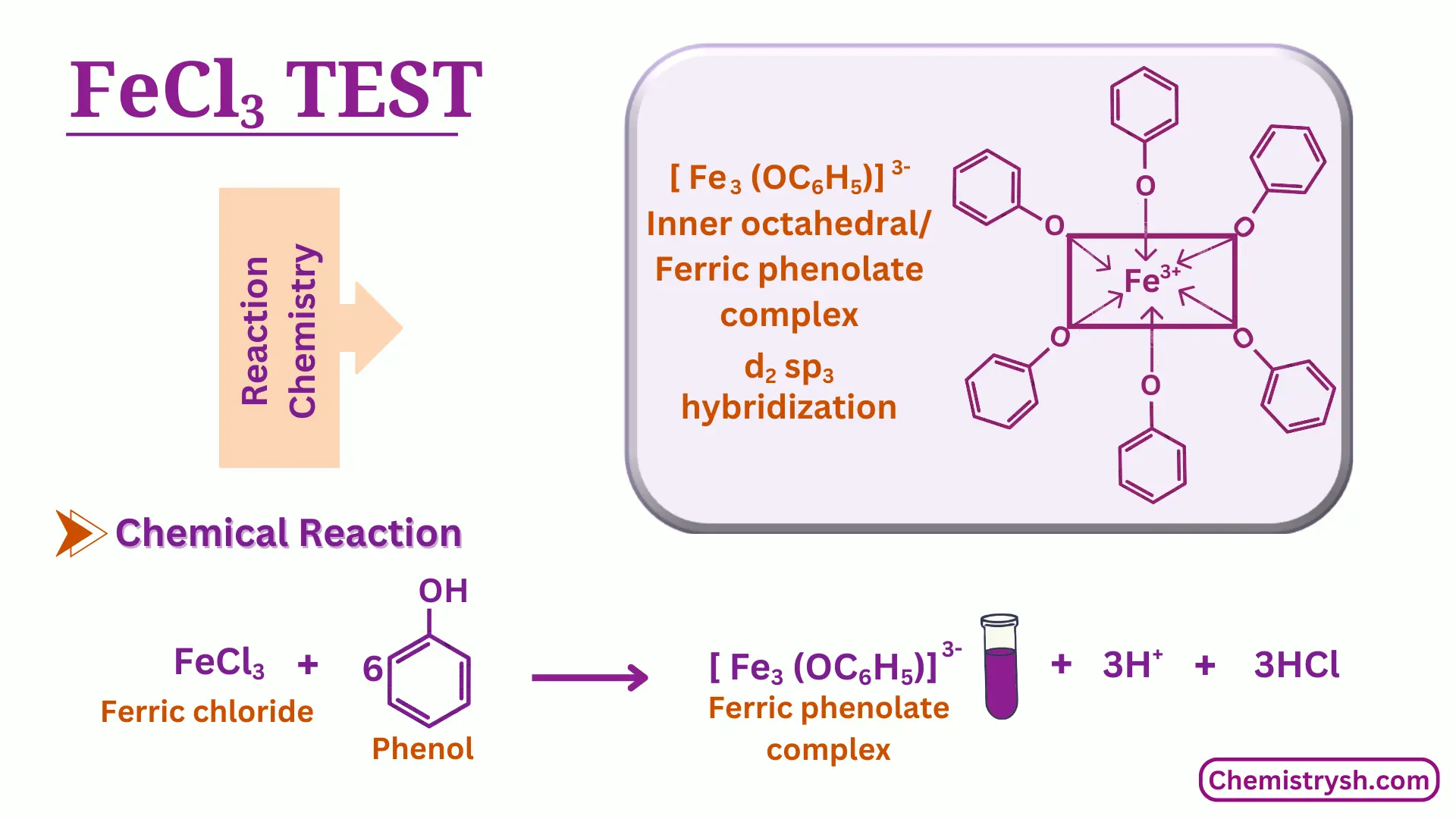
The test is based on the coordination reaction between ferric ions (Fe³⁺) and phenolic hydroxyl groups. Phenols and certain carboxylic acids can react with Fe³⁺ ions to form colored complexes, with different compounds producing different colors. These colors, which may be violet, blue, green, or red depending on the structure of the phenol or carboxylic acid, are used for the identification of various phenols and carboxylic acids.
Historical background
The test is a classical qualitative method dating back to the 19th century. It has been widely used in organic laboratories for rapid phenol detection before the advent of modern spectroscopic techniques.
Chemical Reaction
The general reaction of the ferric chloride test can be represented as:
Fe³⁺ + 6 ArOH → [Fe(ArO)₆]³⁻ + 3 H⁺
Where ArOH represents a phenolic compound or certain carboxylic acids. One ferric ion reacts with six molecules of phenol or carboxylic acid to form a colored coordination complex, which is responsible for the color observed during the test.
Materials Required
- Freshly prepared neutral ferric chloride solution (1–5%)
- Test tubes
- Distilled water (as solvent)
- Sample of phenol or certain carboxylic acid
Quick insight
Why must ferric chloride solution be freshly prepared before use in laboratory tests?
Freshly prepared ferric chloride solution is required because Fe³⁺ ions slowly hydrolyze and precipitate on standing, reducing reagent effectiveness and causing unreliable test results.
Procedure
- Dissolve the test sample in distilled water.
- Add neutral ferric chloride solution dropwise to the sample in the test tube.
- Observe any color change immediately.

Ferric Chloride test with Phenols
Phenols react with freshly prepared ferric chloride solution to form colored complexes, typically violet, blue, or green. This color change occurs due to complex formation between Fe³⁺ ions and the phenoxide ion, confirming the presence of a phenolic –OH group.
Ferric Chloride test with Carboxylic acids
Most carboxylic acids such as acetic, propionic, and benzoic acid do not give a characteristic color with neutral FeCl₃, showing only a pale yellow solution or a brown iron(III) carboxylate precipitate, which is not diagnostic. Compounds containing a phenolic –OH or an enolizable α-hydroxy structure, such as salicylic acid, give a positive violet FeCl₃ test. Any red color observed with simple acids like acetic or formic acid results from ferric carboxylate formation, not true phenolic complexation.
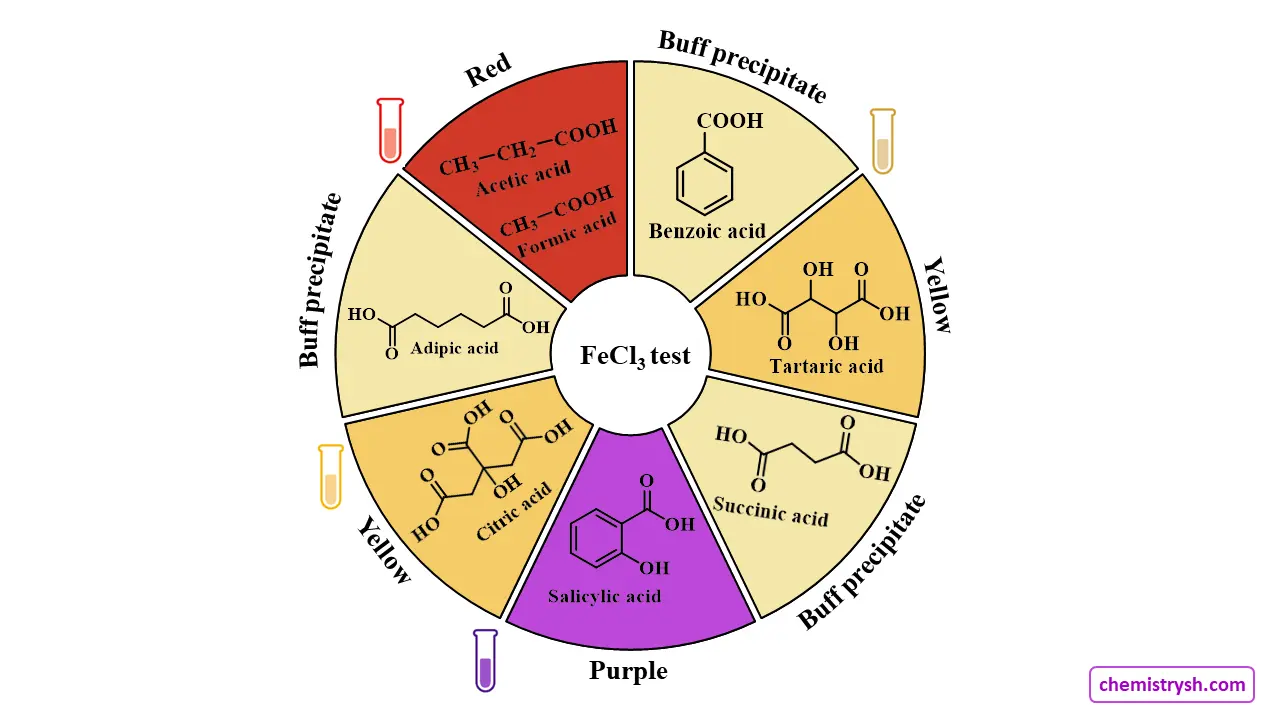
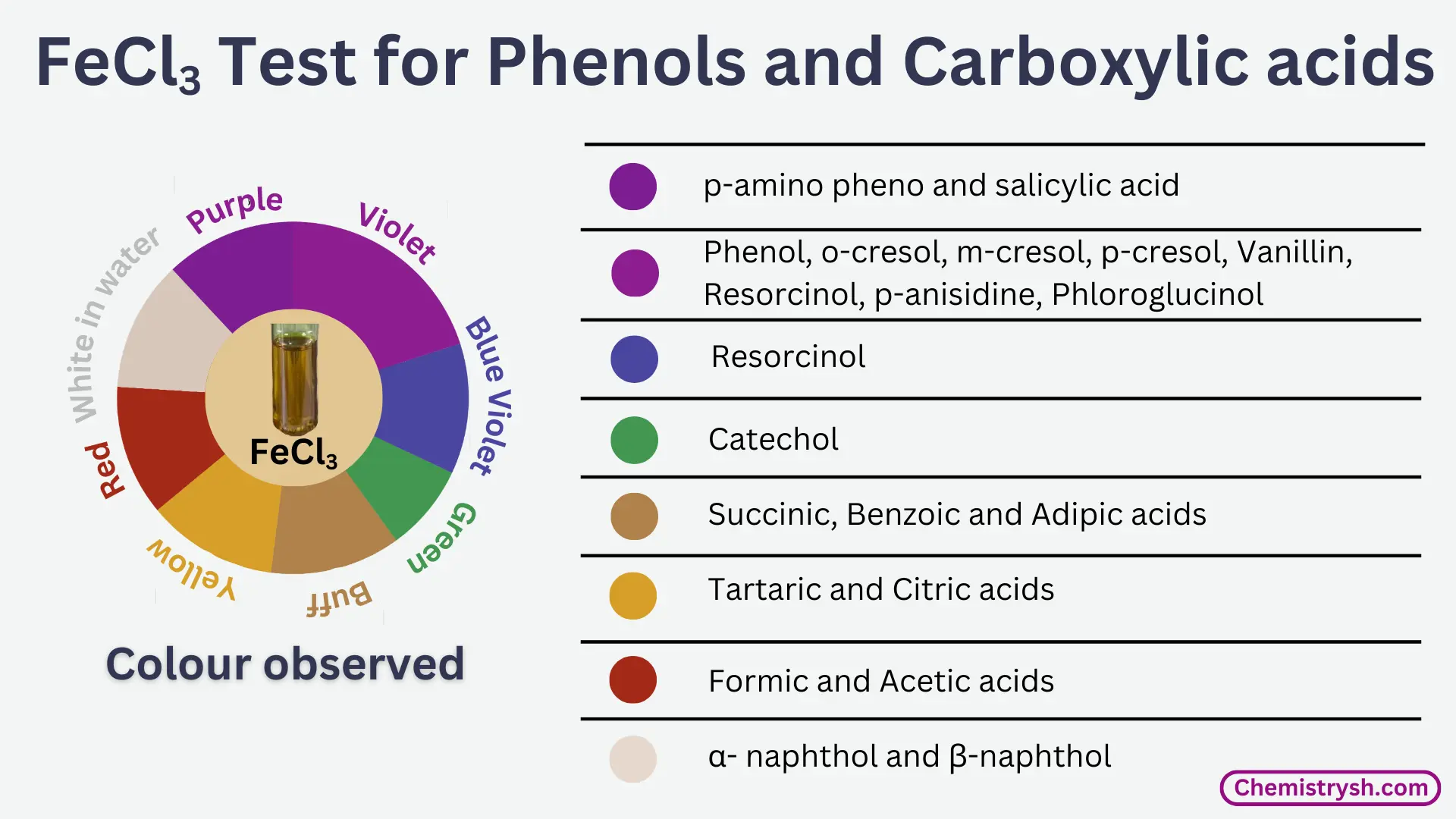
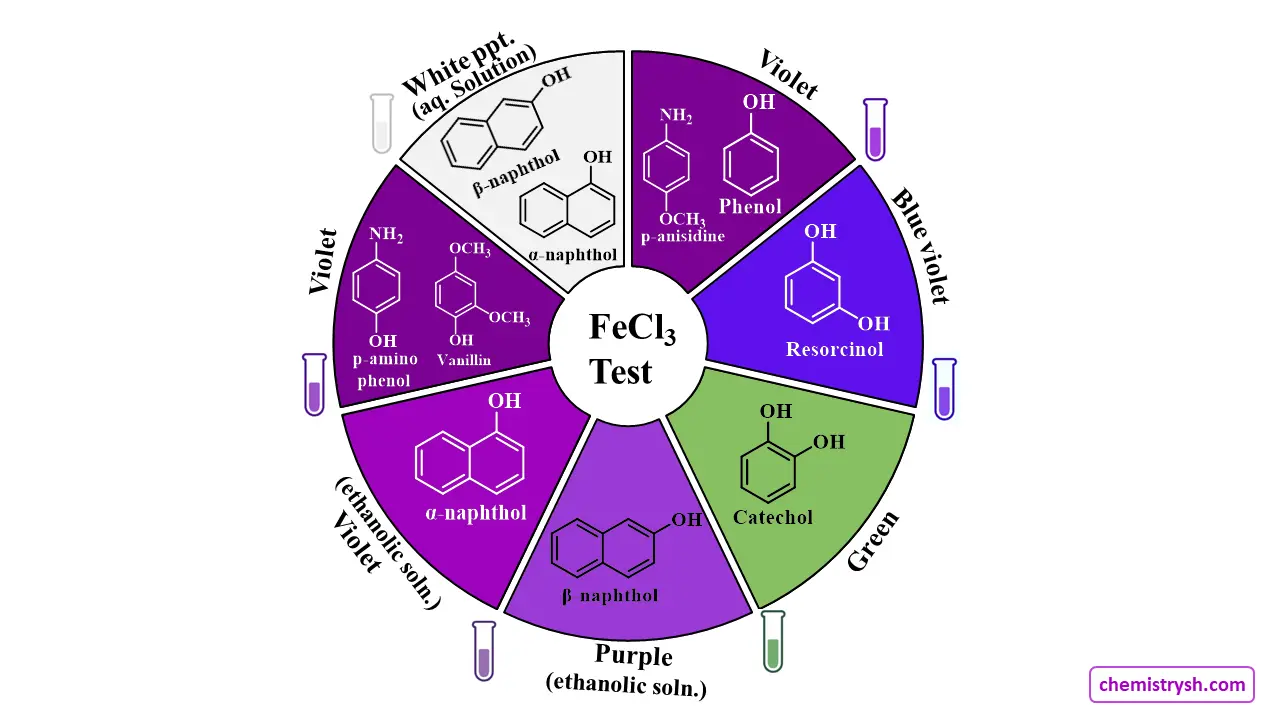
Inference table
|
Colour of Fe³⁺ complex |
Colour Observed |
Inference |
|---|---|---|
|
Buff precipitate |
Adipic, benzoic and succinic acids | |
|
Blue violet |
Resorcinol | |
|
Green |
Catechol | |
|
Purple |
Salicylic acid, p-amino phenol | |
|
Purple in ethanolic solution |
β- naphthol | |
|
Red |
Acetic and Formic acids | |
|
Violet |
Cresols, phenol, phloroglucinol, p-anisidine(amine), resorcinol, vanillin. | |
|
Violet in ethanolic solution |
α- naphthol | |
|
White ppt, if solution prepared was in H2O |
α- and β- Naphthols | |
|
Yellow colouration |
Citric and Tartaric acids |
Applications
- The Ferric Chloride Test is used to quickly detect phenolic and aromatic carboxylic groups in organic compounds.
- It helps in monitoring reactions and identifying functional groups in synthetic and natural samples.
- The test provides a visual confirmation of metal-ligand complex formation, making it a valuable tool for understanding structure-reactivity relationships.
- It is also a useful preliminary check before performing detailed spectroscopic analyses.
Limitations
- FeCl₃ solution must be neutral; acidic solutions may interfere.
- False positives possible with enolic or other reactive compounds.
- Not a quantitative test—only indicates presence.
Precautions
Handle FeCl₃ carefully: it is corrosive; use gloves and avoid inhalation.
Conclusion
The ferric chloride (FeCl₃) test is a reliable qualitative method for identifying phenols through the formation of characteristic colored complexes, typically violet, blue, or green. In contrast, most carboxylic acids do not give a true positive FeCl₃ test, producing only pale yellow solutions or nonspecific precipitates. This clear difference makes the FeCl₃ test an effective tool for distinguishing phenols from carboxylic acids in organic chemistry analysis.
Viva questions
- What is the Ferric Chloride Test used for?
- Explain the principle behind the Ferric Chloride Test.
- Which functional groups give a positive Ferric Chloride Test?
- Why must FeCl₃ solution be neutral?
- What colors indicate a positive test for phenols?
- Can any non-phenolic compounds give a color change? Explain.
- Write the chemical reaction for the Ferric Chloride Test.
- What precautions should be taken while performing this test?
Multiple Choice Questions
MCQ 1
1. The Ferric Chloride Test is used to detect:
A. Ketones
B. Phenols
C. Ketones
D. Aldehydes
MCQ 2
2. Which color indicates a positive test for simple phenols?
A. Red
B. Violet / Blue
C. Green
D. Yellow
MCQ 3
3. Catechol gives which color in the Ferric Chloride Test?
A. Violet
B. Blue
C. Green / Blue-Green
D. Red-Brown
MCQ 4
4. Which of the following functional groups can give a false positive?
A. Alcohols
B. Enols
C. Alkanes
D. Ethers
MCQ 5
5. What is the ain reason for using a neutral FeCl₃ solution?
A. To speed up the reaction
B. To avoid hydrolysis and precipitation
C. To enhance color intensity
D. No effect
MCQ 6
6. Which form of ferric chloride is used in this test?
A. Solid FeCl₃
B. Alcoholic FeCl₃
C. Aqueous neutral FeCl₃
D. Concentrated FeCl₃
FAQ’s
References of FeCl3Test
- Vogel, A. I. Vogel’s Textbook of Practical Organic Chemistry, 5th Edition.
- Furniss, B. S., Hannaford, A. J., Smith, P. W. G., & Tatchell, A. R. Vogel’s Textbook of Practical Organic Chemistry, 4th Edition.
- Mann, F. G., & Saunders, B. C. Practical Organic Chemistry.
- Nichols, D. E. Organic Chemistry Lab Techniques.