Melting point determination is an important procedure in chemistry laboratory. The melting point is a fundamental physical property of matter. It refers to the specific temperature at which a solid substance changes into a liquid under standard atmospheric pressure. At this temperature, both solid and liquid phases exist in equilibrium.
In chemistry, the melting point is widely used to identify substances, check purity, and study intermolecular forces. It is especially important in organic chemistry and laboratory analysis.
What Is Melting Point?
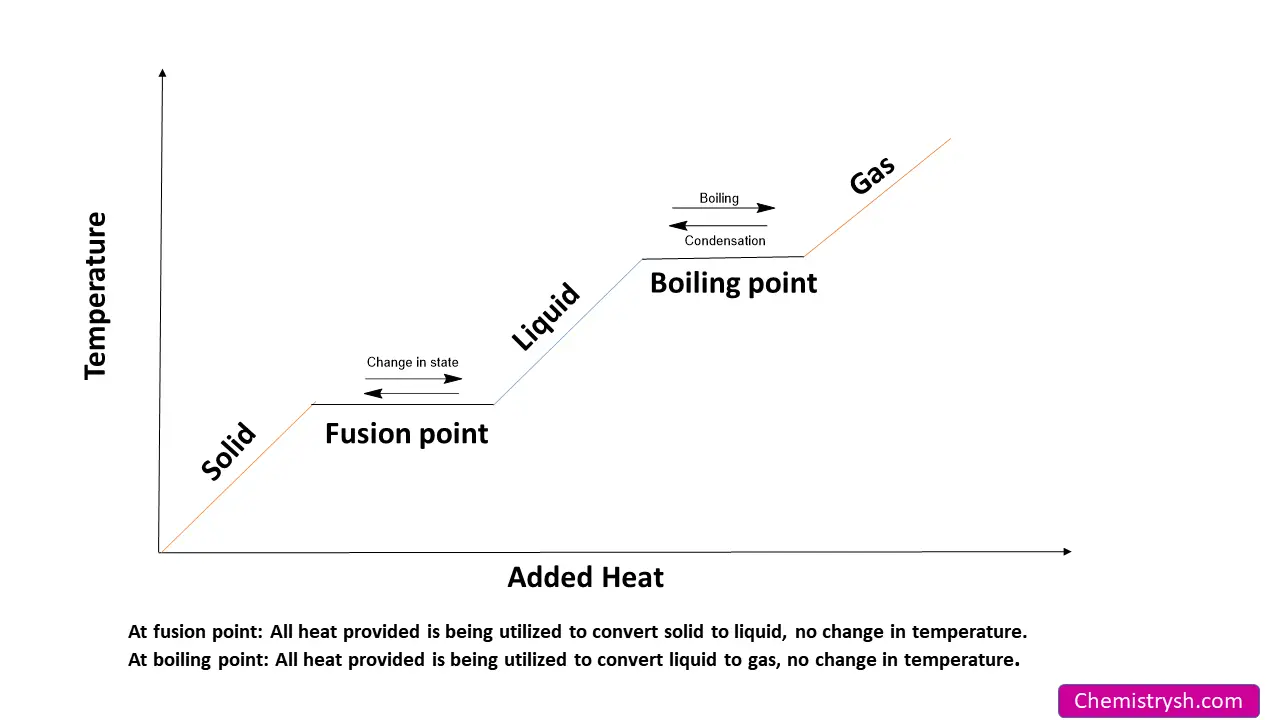
The melting point is the temperature at which the orderly structure of a solid breaks down and turns into a liquid. During melting, the supplied heat does not raise the temperature. Instead, it is used to overcome the forces holding the particles in fixed positions.
Each pure substance has a characteristic melting point. This value remains constant if the substance is pure and the pressure is unchanged. Because of this consistency, melting point data is used as an identification tool in chemistry.
Melting Point and Freezing Point
The melting point and freezing point occur at the same temperature for a pure substance. The difference lies in the direction of the phase change. Melting occurs when a solid absorbs heat. Freezing occurs when a liquid loses heat.
Although the temperatures are the same, the processes are opposite. In laboratory practice, melting point is usually discussed for solids, while freezing point is discussed for liquids.
Heating Curve for Melting Point
Scientific Basis of Melting
Melting is a phase transition from solid to liquid. In solids, particles vibrate around fixed positions. As heat is supplied, vibration increases. At the melting point, particles gain enough energy to overcome intermolecular forces.
The energy required to change a solid into a liquid without changing temperature is called the heat of fusion. Stronger intermolecular forces require more energy, which results in a higher melting point.
Factors Affecting Melting Point
1. Intermolecular Forces
- Strong intermolecular forces increase the melting point.
- Substances with hydrogen bonding or ionic bonds usually have high melting points.
2. Molecular Structure and Melting Behavior
- Symmetrical molecules:
Pack efficiently in the crystal lattice due to their regular shape, producing stronger intermolecular forces and a higher, sharp melting point.
Example: p-Dichlorobenzene melts at a higher temperature and more sharply than its unsymmetrical isomers. - Large or irregular molecules:
Pack poorly because of their bulky or uneven structure, resulting in weaker lattice forces and a wider range of melting point rather than a sharp melting point.
Examples: o-Dichlorobenzene and branched organic compounds melt over a broader temperature interval.
3. Purity
- Pure substances melt at a definite temperature.
- Impurities disturb the crystal structure and lower the melting point.
Did you know?
Ice melts at 0 °C, but sea ice melts at a lower temperature due to dissolved salts.
Melting Point Range and Purity
1. Sharp Melting Point
- A sharp melting point is observed for a pure substance.
- The solid melts at one fixed temperature.
- It indicates high purity and uniform crystal structure.
2. Range of Melting Point
- A melting point range is observed for an impure substance.
- Melting occurs over a range of temperatures, not at a single point.
- It indicates the presence of impurities or poor crystallization.
- A difference of just 2 °C in melting point can indicate impurity in an organic compound.
Methods of Determining Melting Point
The most common laboratory method is the capillary tube method. A small amount of finely powdered sample is placed in a thin glass capillary tube. The tube is heated gradually in a melting point apparatus.
The temperature at which melting begins and the temperature at which the sample completely liquefies are recorded. Accurate melting point determination requires slow heating near the expected melting point.
Modern digital melting point apparatus provides precise readings, but the basic principle remains the same.
Classical Method (Beaker / Liquid Bath Method)
Sample preparation:
A small amount of the finely powdered solid is packed into a thin glass capillary tube sealed at one end.
Setting the liquid bath:
A beaker is filled with a suitable heating liquid (water for low melting substances, oil for higher melting substances) and placed on a heat source.
Assembly of apparatus:
The capillary tube is attached to a thermometer so that the sample is close to the thermometer bulb, and both are immersed in the liquid bath.
Gradual heating:
The liquid in the beaker is heated slowly, especially near the expected melting point, to allow accurate temperature measurement.
Observation of melting:
The temperature at which the solid first starts melting and the temperature at which it completely liquefies are carefully noted.
Recording the result:
The melting point is reported as the range of melting point, from the beginning to the completion of melting.

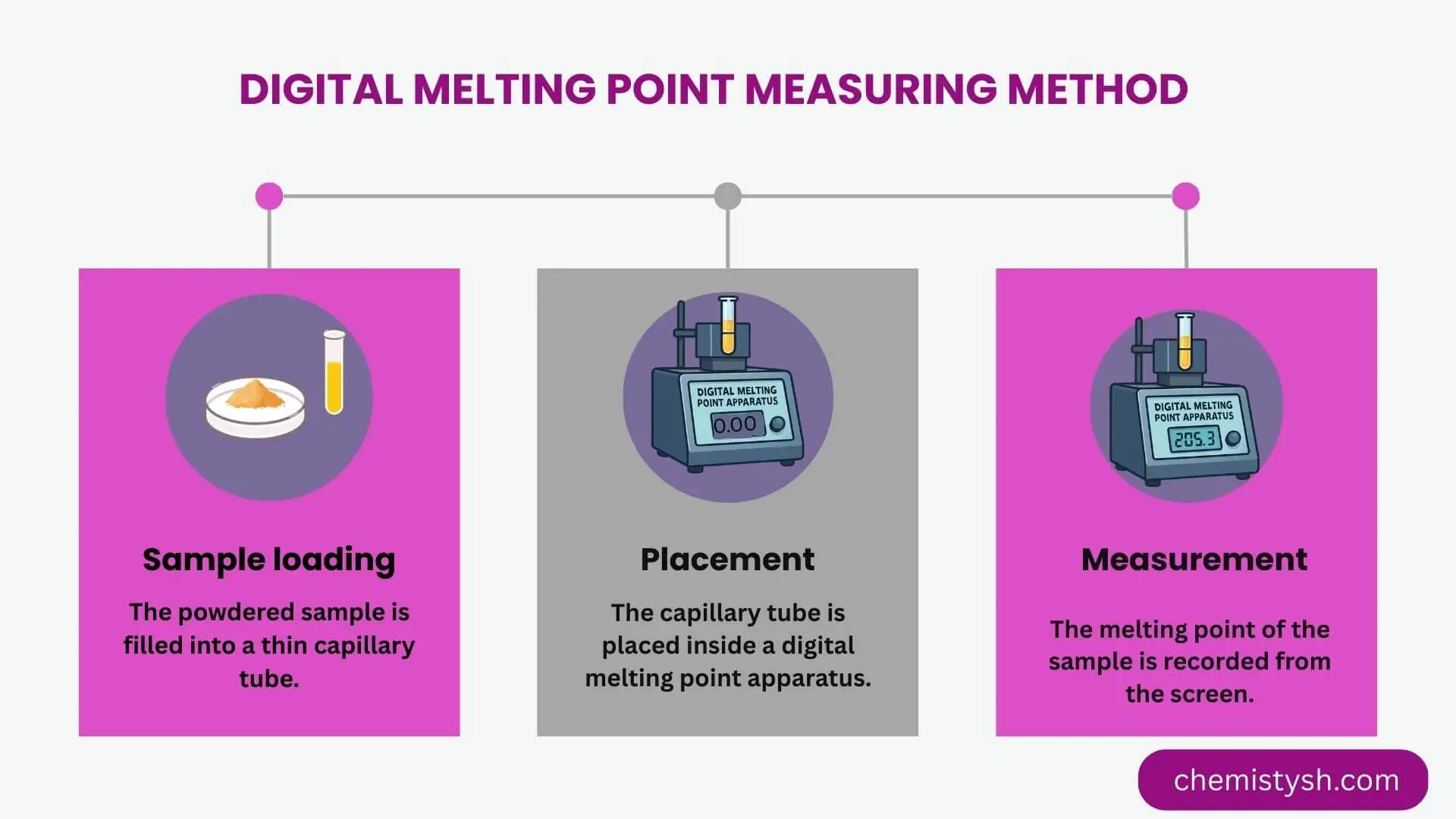
Digital Method (Digital Melting Point Apparatus)
Sample loading:
A small quantity of the powdered sample is filled into a capillary tube.
Insertion into apparatus:
The capillary tube is placed into the sample holder of the digital melting point apparatus.
Setting parameters:
The heating rate is selected according to the expected melting point of the substance.
Automatic heating:
The instrument heats the sample in a controlled and uniform manner.
Detection of melting:
The apparatus automatically detects the start and completion of melting or allows visual observation through a magnifying lens.
Display of result:
The melting point or range of melting point is displayed digitally with high accuracy
Melting Point vs Boiling Point
The melting point refers to the transition from solid to liquid. The boiling point refers to the transition from liquid to gas. Both are physical properties, but they describe different phase changes.
Melting point depends mainly on solid-state forces. Boiling point depends on forces between liquid molecules and vapor pressure. Because of this difference, a substance can have a low melting point but a high boiling point.

Importance of Melting Point in Chemistry
Melting point determination is widely used in organic chemistry to identify unknown compounds. It is also used to confirm the identity of synthesized substances.
In pharmaceutical and industrial chemistry, melting point is used for quality control. It helps detect impurities and ensures consistency of raw materials and finished products.
Conclusion
The melting point is a simple yet powerful physical property in chemistry. It provides valuable information about substance identity, purity, and molecular structure.
Because of its reliability and ease of measurement, melting point determination remains an essential technique in chemical laboratories and educational settings.
Viva questions
- Define melting point.
- How can measuring the melting point help identify a compound?
- What is meant by a sharp melting point?
- What is the difference between a sharp melting point and a broad melting point?
- Which types of compounds generally have high melting points? Give examples.
Multiple Choice Questions
MCQ 1
1. Which of the following best defines melting point?
A. Temperature at which a solid vaporizes
B. Temperature at which a solid turns into a liquid at equilibrium
C. Temperature at which a liquid boils
D. Temperature at which a gas condenses
MCQ 2
2. A pure solid usually shows:
A. Low melting point and broad range
B.High melting point and broad range
C. Sharp melting point over a narrow temperature range
D. Melting point unaffected by impurities
MCQ 3
3. Impurities in a solid generally cause:
A. Increase in melting point
B. Sharp melting point
C. Decrease in melting point and wider melting range
D. No effect on melting behavior
FAQs
References of Melting point
- Vogel, A. I. Textbook of Practical Organic Chemistry. Longman.
- Furniss, B. S., Hannaford, A. J., Smith, P. W. G., & Tatchell, A. R. Vogel’s Practical Organic Chemistry. Pearson Education.
- Mann, F. G., & Saunders, B. C. Practical Organic Chemistry. Pearson.
- Shriner, R. L., Fuson, R. C., Curtin, D. Y., & Morrill, T. C. The Systematic Identification of Organic Compounds. Wiley.